 |
|
Bios |
Anti-VEGF intravitreal therapy has revolutionized treatment of exudative macular degeneration, diabetic macular edema, proliferative diabetic retinopathy and retinal vascular occlusion. Its efficacy in treating cystoid macular edema, choroidal neovascularization and retinal neovascularization associated with these diseases is backed by large controlled clinical trials.
Ocular inflammation may be associated with a number of anatomic indications for anti-VEGF agents, including CME, CNV and RNV. This can be due to either active inflammatory disease or secondary structural complications that result from retinal ischemia, chorioretinal scarring or other predisposing factors.1
Paucity of evidence in uveitis
While the efficacy of anti-VEGF therapy for exudative macular degeneration, diabetic retinal disease and retinal vascular occlusion has been well established, treatment is more difficult to study systematically in uveitis-related ocular inflammation because of the diversity of uveitic diseases and their varied pathophysiologic mechanisms.
Uveitis specialists rely on case reports, case series and clinical judgment to determine how and when to use anti-VEGF therapy for uveitis, much of which is off-label. This article will discuss the available evidence, such as it is, as well as clinical examples of the use of intravitreal anti-VEGF therapy in uveitis.
Inflammatory control is imperative
Uncontrolled inflammation in uveitis is usually the direct cause of uveitic CME and, occasionally, the direct cause of CNV or RNV. Even in the absence of ischemia, inflamed eyes can develop CNV or RNV, which may be successfully treated with inflammatory control alone.2-4 Additionally, inflammatory control in occlusive retinal vasculitis may prevent future ischemic RNV, and control of uveitis associated with chorioretinitis may prevent future scar-associated CNV.
After infectious causes have been ruled out or treated, uveitis quiescence must be achieved with local or systemic corticosteroids and may require additional immunosuppressive agents.
Quiet disease may vary in appearance depending on the uveitic type. Slit lamp or indirect biomicroscopy may show improvement in cell and haze or in the morphology and number of chorioretinal lesions. Other signs of disease quiescence may include imaging findings such as improvement in choroidal or retinal thickness by optical coherence tomography; normalization of abnormal fundus autofluorescence; or reduction in vascular leakage or ischemia by indocyanine green angiography or fluorescein angiography may be (Figure 1).
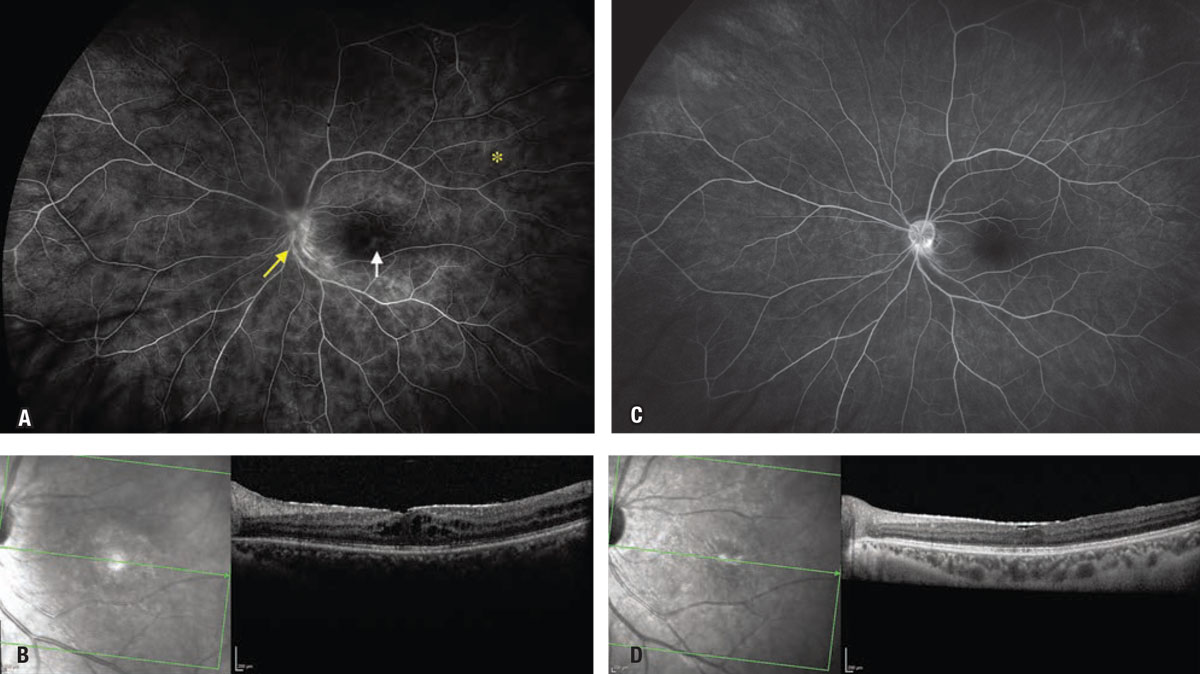 |
| Figure 1. A) Late-phase fluorescein angiography of a 25-year-old patient with active chronic intermediate uveitis showing diffuse small vessel non-occlusive retinal vasculitis (asterisk) and disc leakage (yellow arrow) with associated cystoid macular edema (white arrow). B) Optical coherence tomography image of the patient’s CME. C) Late-phase FA of the same patient after uveitis control was achieved via treatment with intravitreal corticosteroid shows resolution of the CME and small-vessel vasculitis, but mild residual disc leakage. D) OCT of the patient’s retina after CME resolution. An epiretinal membrane and retinal atrophy are structural consequences of previous chronic disease activity. |
Retinal neovascularization
RNV is an infrequent but clinically important complication of uveitis. RNV is significantly more common in uveitis with occlusive vasculitis, such as infectious necrotizing retinitis, systemic lupus erythematosus, Beçhet’s disease, idiopathic retinal vasculitis with neuroretinitis (known as IRVAN), tuberculosis-associated retinal vasculitis or Eales disease.5 Other risk factors include cigarette smoking and young age (<35 years).6 In these cases, RNV is often a sign of active inflammation.
Only a few case reports and case series have reviewed the use of anti-VEGF agents for uveitis-associated RNV.1,7-9 Complete or partial regression of neovascularization was reported in most of these cases. Bevacizumab (Avastin, Genentech/Roche) has been shown to be effective in treating a recalcitrant uveitic RNV after scatter laser photocoagulation (SLP).11
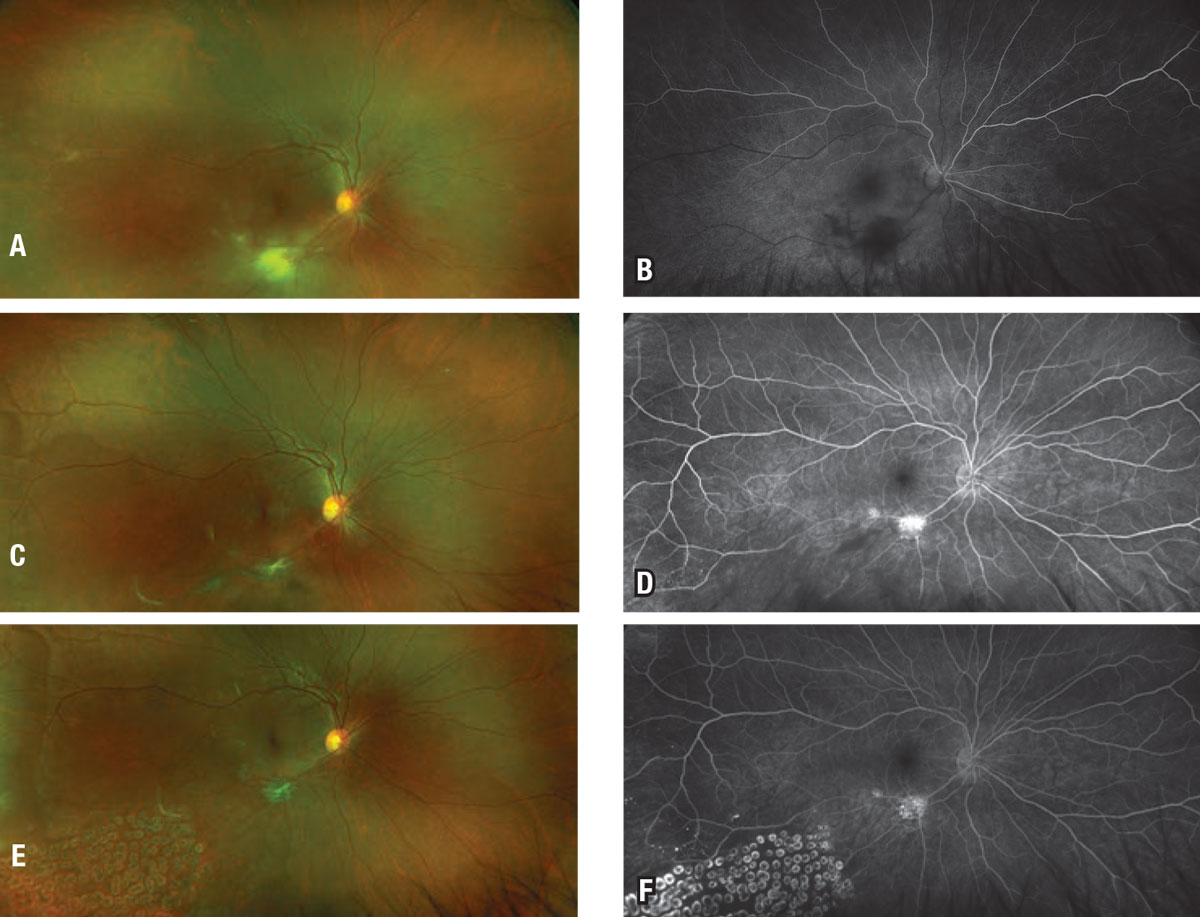 |
| Figure 2. A) Pseudocolor fundus photo of a 32-year-old patient with primary acquired toxoplasmosis retinochoroiditis with an associated occlusive arteriolar vasculitis resulting in an inferior branch retinal artery occlusion. B) Early phase fluorescein angiography demonstrates a large area of inferotemporal retinal nonperfusion. C) Pseudocolor fundus photo after treatment with oral trimethoprim-sulfamethoxazole and oral prednisone demonstrates resolution of the retinochoroiditis and new retinal neovascularization with vitreous hemorrhage. D) Late-phase FA demonstrates an area of RNV. E,F) Pseudocolor image and FA of RNV regression and resolved vitreous hemorrhage after scatter laser photocoagulation and intravitreal bevacizumab. |
However, clinicians should exercise caution in highly ischemic eyes. This is because anti-VEGF treatments may, in rare cases, close off thread-like vessels providing vital perfusion, paradoxically worsening retinal ischemia. Further study is needed to determine whether SLP, isolated anti-VEGF or combined techniques work best in uveitic RNV (Figure 2).
Choroidal neovascularization
In posterior uveitis and panuveitis, CNV has an incidence and prevalence in the 2 percent range,12 but it’s a hallmark of punctate inner choroiditis (PIC) and multifocal choroiditis (MFC), occurring in up to half of cases.13 Identifying and treating uveitic CNV can be challenging for two reasons: because neovascular membranes and inflammatory lesions at the level of the retinal pigment epithelium-Bruch’s membrane (RPE-BM) complex can have similar characteristics on imaging; and because angiogenesis may occur in the setting of direct uveitic involvement of the RPE-BM and/or as a result of previous RPE-BM degenerative disruption.1
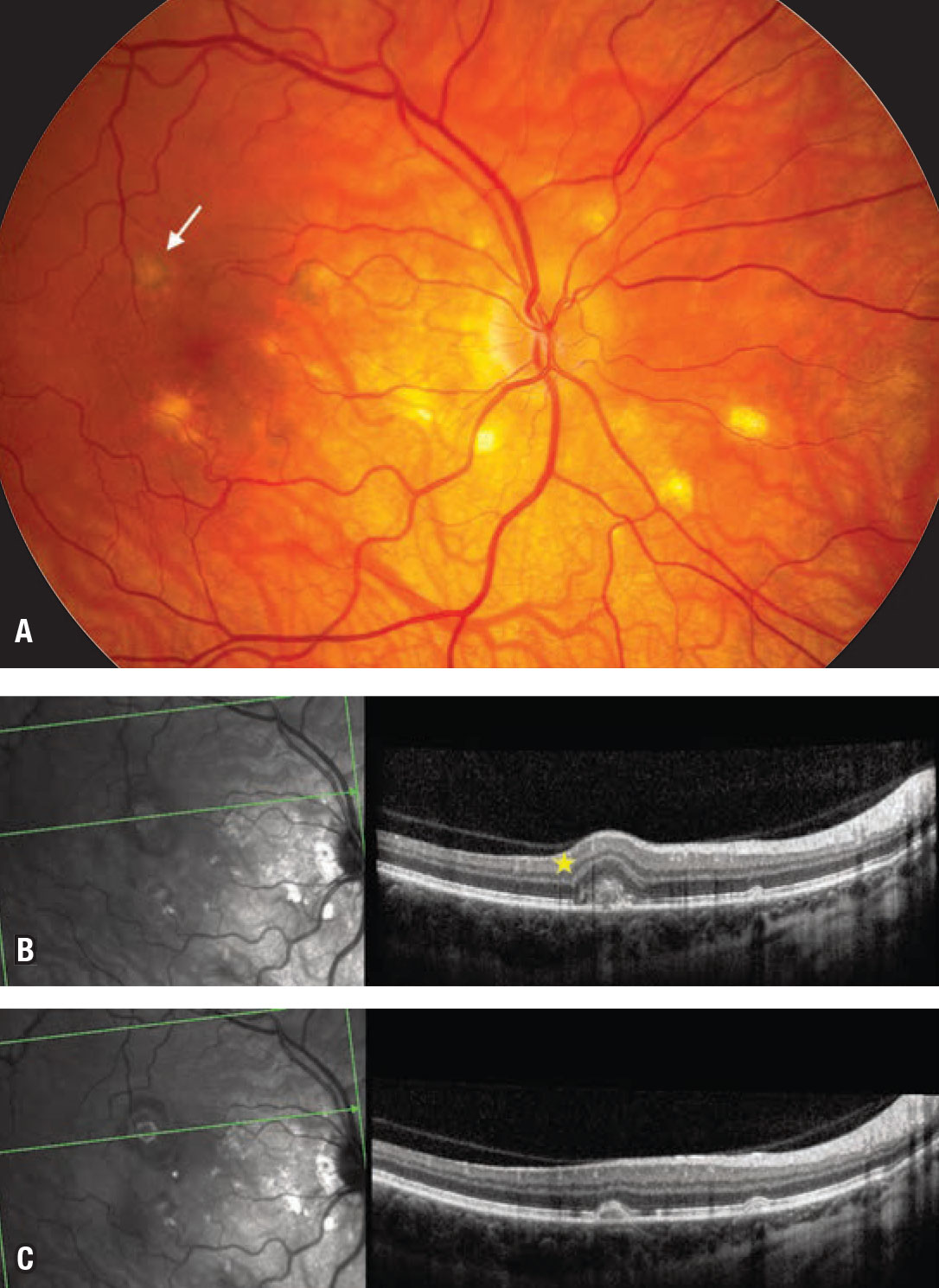 |
| Figure 3. A) Color fundus photo of a 43-year-old myopic female with punctate inner choroiditis who noticed an increase in one of her small blind spots in the inferonasal sector, which manifested as a greenish gray subretinal lesion (arrow). B) Optical coherence tomography reveals a choroidal neovascular membrane with heterogeneous dome-shaped subretinal hyperreflective material and ellipsoid zone disruption, and a break in Bruch’s layer with choroidal hyper-transmission, indicating retinal pigment epithelium/photoreceptor disruption (star). C) An inactive lesion in the nasal macula. After a series of three intravitreal bevacizumab injections four weeks apart, the choroidal neovascularization became inactive and symptoms resolved. |
Advanced multimodal imaging analysis and OCT-angiography are useful for evaluating uveitic CNV (Figure 3).14-16
Angiogenesis in CNV is a complex process in which inflammatory mediators play a critical role.17 In some cases of uveitic CNV, corticosteroid and anti-VEGF treatments may have overlapping therapeutic effects.
Multiple case studies and series have shown that uveitic CNV frequently responds to intravitreal anti-VEGF injections, often administered concomitantly with appropriate antimicrobials or with short-acting local or systemic corticosteroids where appropriate for additional effect.13,18
In patients with noninfectious posterior uveitis with frequent CNV recurrences, long-term immunosuppression may be useful in decreasing therapeutic dependence on anti-VEGF agents.
Cystoid macular edema
The reported prevalence of uveitic CME ranges from 20 to 70 percent, and it may be more common in chronic or intermediate uveitis, and more so in adults than in children.20
Uveitic CME in non-infectious disease is typically treated with local and systemic corticosteroids as well as with steroid-sparing immunomodulatory therapy.21 Oral carbonic anhydrase inhibitors and topical non-steroidal anti-inflammatory drops may be used as adjunctive therapy.22
 |
In cases of recalcitrant CME or when local or systemic toxicities limit the use of corticosteroids, the need for additional therapy arises. Patients with uveitic CME have increased aqueous levels of vascular endothelial growth factor compared with unaffected uveitis patients,23 making VEGF a target of interest in uveitis.
Intravitreal anti-VEGF therapy with bevacizumab may result in an improvement in retinal thickness and visual acuity in uveitic eyes.24–26 However, the beneficial effect is generally transient;24 it often dissipates within a month.27
Patients with isolated petaloid fluorescein leakage (consistent with controlled uveitis) have a more favorable response to anti-VEGF therapy than those with more extensive ocular inflammation as evidenced by leakage of the choroid and optic nerve.24
A word of caution
Drug-induced uveitis has been reported rarely with the use of bevacizumab, ranibizumab (Lucentis, Genentech/Roche) and aflibercept (Eylea, Regeneron Pharmaceuticals), with the incidence likely in the 1 percent range.28 However, their use is generally considered to be safe in uveitis. Brolucizumab (Beovu, Novartis) is associated with a small but significant risk of intraocular inflammation and retinal vasculitis,29,30 and should be avoided in uveitic eyes.
Bottom line
Intravitreal anti-VEGF agents may be used successfully in select cases of uveitis with inflammatory CNV, RNV and recalcitrant uveitic CME, but the quality of evidence supporting their efficacy is low,1 so decisions must often be made based on limited evidence and clinical judgment.
When considering anti-VEGF treatment in uveitis, concomitant treatment to control the underlying inflammatory or infectious disease is critical. In uveitic eyes with RNV, consider laser photocoagulation to areas of ischemic retina with adjunctive anti-VEGF therapy for residual neovascularization.
While no studies provide guidance on the choice of specific anti-VEGF agents in uveitis, brolucizumab should likely be avoided in inflamed eyes due to its increased risk of intraocular inflammation and occlusive retinal vasculitis. RS
REFERENCES
1. Gulati N, Forooghian F, Lieberman R, Jabs DA. Vascular endothelial growth factor inhibition in uveitis: A systematic review. Br J Ophthalmol. 2011;95:162-1650.
2. Sanislo SR, Lowder CY, Kaiser PK, et al. Corticosteroid therapy for optic disc neovascularization secondary to chronic uveitis. Am J Ophthalmol. 2000;130:724-731.
3. Flaxel CJ, Owens SL, Mulholland B, Schwartz SD, Gregor ZJ. The use of corticosteroids for choroidal neovascularisation in young patients. Eye (Lond). 1998;12( Pt 2):266-272.
4. Dees C, Arnold JJ, Forrester JV, Dick AD. Immunosuppressive treatment of choroidal neovascularization associated with endogenous posterior uveitis. Arch Ophthalmol. 1998;116:1456-1461.
5. Talat L, Lightman S, Tomkins-Netzer O. Ischemic retinal vasculitis and its management. J Ophthalmol. 2014;2014:197675. Published online April 15, 2014. doi: 10.1155/2014/197675.
6. Patel AK, Newcomb CW, Liesegang TL, et al, Systemic Immunosuppressive Therapy for Eye Diseases Research Group. Risk of retinal neovascularization in cases of uveitis. Ophthalmology. 2016;123:646-654.
7. Küçükerdönmez C, Akova YA, Yilmaz G. Intravitreal injection of bevacizumab in Eales disease. Ocul Immunol Inflamm. 2008;16:63-650.
8. Kumar A, Sinha S. Rapid regression of disc and retinal neovascularization in a case of Eales disease after intravitreal bevacizumab. Can J Ophthalmol. 2007;42:335–336.
9. Chaudhary KM, Mititelu M, Lieberman RM. An evidence-based review of vascular endothelial growth factor inhibition in pediatric retinal diseases: Part 2. Coats’ disease, best disease, and uveitis with childhood neovascularization. J Pediatr Ophthalmol Strabismus. 2013;50:11-19.
10. Patel AK, Newcomb CW, Liesegang TL, et al. Risk of retinal neovascularization in cases of uveitis. Ophthalmology. 2016;123:646-654.
11. Kurup S, Lew J, Byrnes G, Yeh S, Nussenblatt R, Levy-Clarke G. Therapeutic efficacy of intravitreal bevacizumab on posterior uveitis complicated by neovascularization. Acta Ophthalmologica. 2009;87:349-352.
12. Baxter SL, Pistilli M, Pujari SS, et al. Risk of choroidal neovascularization among the uveitides. Am J Ophthalmol. 2013;156:468-477.e2.
13. Cunningham ET Jr, Pichi F, Dolz-Marco R, Freund KB, Zierhut M. Inflammatory choroidal neovascularization. Ocul Immunol Inflamm. 2020;28:2-6.
14. Hoang QV, Cunningham ET Jr, Sorenson JA, Freund KB. The “pitchfork sign” a distinctive optical coherence tomography finding in inflammatory choroidal neovascularization. Retina. 2013;33:1049–1055
15. Spaide RF, Goldberg N, Freund KB. Redefining multifocal choroiditis and panuveitis and punctate inner choroidopathy through multimodal imaging. Retina. 2013;33:1315–1324
16. Baumal CR, de Carlo TE, Waheed NK, Salz DA, Witkin AJ, Duker JS. Sequential optical coherence tomographic angiography for diagnosis and treatment of choroidal neovascularization in multifocal choroiditis. JAMA Ophthalmol. 2015;133:1087-90.
17. Wang T, Tsirukis DI, Sun Y. Targeting neuroinflammation in neovascular retinal diseases. Front Pharmacol. 2020;11:234.
18. Mansour AM, Mackensen F, Mahendradas P, Khairallah M, Lai TY, Bashshur Z. Five-year visual results of intravitreal bevacizumab in refractory inflammatory ocular neovascularization. Clin Ophthalmol. 2012;6:1233-1237.
19. Cerquaglia A, Fardeau C, Cagini C, Fiore T, LeHoang P. Inflammatory choroidal neovascularization: Beyond the intravitreal approach. Ocul Immunol Inflamm. 2018; 26:1047-1052
20. Accorinti M, Okada AA, Smith JR, Gilardi M. Epidemiology of macular edema in uveitis. Ocul Immunol Inflamm. 2019;27:169-180.
21. Tomkins-Netzer O, Lightman SL, Burke AE, et al; Multicenter Steroid Treatment Trial and Follow-up Study Research Group. Seven-year outcomes of uveitic macular edema: The Multicenter Uveitis Steroid Treatment Trial and follow-up study results. Ophthalmology. 2021;128:719-728.
22. Pepple KL, Nguyen MH, Pakzad-Vaezi K, et al. Response of inflammatory cystoid macular edema to treatment using oral acetazolamide. Retina. 2019;39:948-955.
23. Fine HF, Baffi J, Reed GF, Csaky KG, Nussenblatt RB. Aqueous humor and plasma vascular endothelial growth factor in uveitis-associated cystoid macular edema. Am J Ophthalmol. 2001;132:794-796.
24. Weiss K, Steinbrugger I, Weger M, et al. Intravitreal VEGF levels in uveitis patients and treatment of uveitic macular oedema with intravitreal bevacizumab. Eye (Lond). 2009;23:1812-1818.
25. Cordero Coma M, Sobrin L, Onal S, Christen W, Foster CS. Intravitreal bevacizumab for treatment of uveitic macular edema. Ophthalmology. 2007;114:1574-1579.e1.
26. Reddy AK, Cabrera M, Yeh S, Davis JL, Albini TA. Optical coherence tomography-guided ranibizumab injection for cystoid macular edema in well-controlled uveitis: twelve-month outcomes. Retina. 2014;34:2431-2438.
27. Ziemssen F, Deuter CM, Stuebiger N, Zierhut M. Weak transient response of chronic uveitic macular edema to intravitreal bevacizumab (Avastin). Graefes Arch Clin Exp Ophthalmol. 2007;245:917-918.
28. Iqbal KM, Hay MW, Emami-Naeini P. Medication-induced uveitis: An update. J Ophthalmic Vis Res. 2021;16:84-92.
29. Monés J, Srivastava SK, Jaffe GJ, et al. Risk of inflammation, retinal vasculitis, and retinal occlusion-related events with brolucizumab: Post hoc review of HAWK and HARRIER. Ophthalmology. 2021;128:1050-1059.
30. Baumal CR, Spaide RF, Vajzovic L, et al. Retinal vasculitis and intraocular inflammation after intravitreal injection of brolucizumab. Ophthalmology. 2020;127:1345-1359.




