 |
 |
A 71-year-old woman was referred to the University of Washington Medicine Eye Institute after receiving a new diagnosis of age-related macular degeneration by an outside provider. She denied any acute changes in her vision. Her ocular history only included upper-lid blepharoplasties. Her medical history was unremarkable and she wasn’t taking any systemic medications.
Ocular examination findings
On presentation, best-corrected Snellen visual acuity was 20/20 OD and 20/30 OS. Her pupils were equal and reactive without relative afferent pupillary defect, and her intraocular pressure was within normal limits in each eye. Confrontational visual fields and extraocular movements were full in both eyes. Her slit lamp exam was notable only for bilateral mild nuclear sclerosis.
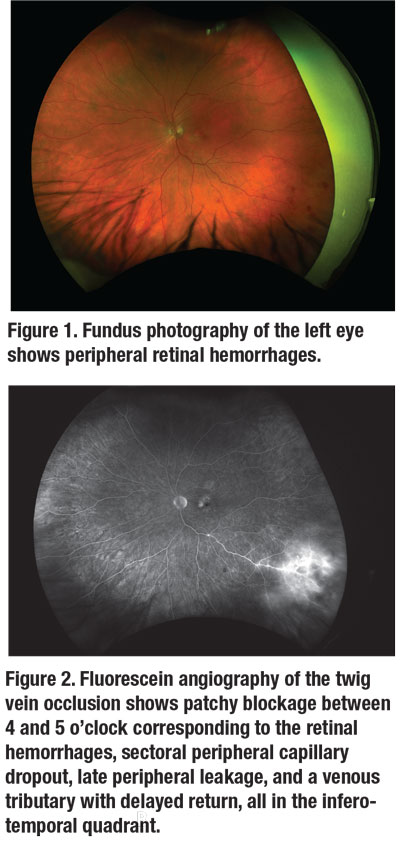
The dilated fundus examination revealed macular pigmentary changes and drusen in both eyes. The far periphery of the left eye had several blot hemorrhages at 4 o’clock.
 |
Findings on imaging
To further evaluate the focal peripheral retinal hemorrhages, we obtained color fundus photographs (Figure 1) and widefield fluorescein angiography images.
FA of the left eye (Figure 2) demonstrated leakage on transit within the macula, consistent with a choroidal neovascular membrane from neovascular AMD. Optical coherence tomography imaging of the macula also demonstrated the CNVM with subretinal fluid associated with drusen.
The FA also showed patchy blockage of fluorescence between 4 and 5 o’clock corresponding to the retinal hemorrhages, sectoral peripheral capillary dropout, late peripheral leakage, and a venous tributary with delayed return, all in the inferotemporal quadrant.
Diagnosis and management
We diagnosed exudative macular degeneration based on the leakage pattern on FA and OCT. The patient responded well to serial intravitreal anti-VEGF injections.
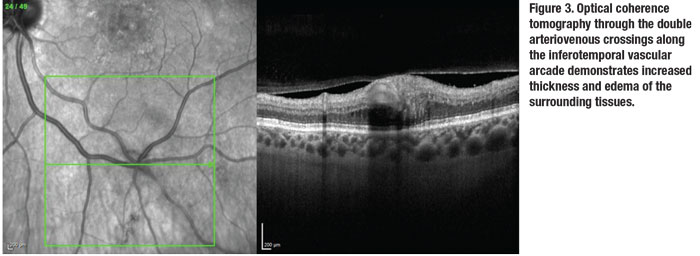
Additionally, given the pattern of intraretinal hemorrhages and delayed return in an isolated venous tributary, we diagnosed an incidental retinal vein tributary, or “twig,” occlusion. Interestingly, the juncture of the venous occlusion seemed to include not one but two arteriovenous (AV) crossings given its location at the branch point of both the arteries and the veins. We also obtained OCT through the AV crossings (Figure 3).
Because of the peripheral location of the twig retinal vein occlusion and the lack of other accompanying symptoms, we recommended observation. We also counseled the patient to follow up with her primary-care provider to optimize her cardiovascular health. She continued to follow up at our institution for the care of her AMD with no further complications attributable to her vein occlusion.
 |
 |
Etiology of ‘twigs’
RVO is one of the most common types of retinal vascular disease.1 It can be classified by the extent of the occlusion, with central vein occlusion being the most severe, followed by hemiretinal vein occlusion and branch RVO, which can further be subdivided based on involvement of first- or second-order vein tributaries. The latter includes central (macular) tributaries or, in the case of our patient, peripheral “twig” occlusions. These peripheral occlusions disrupt the least amount of retinal surface area and are accordingly less symptomatic.2
Like other types of vein occlusions, the mechanism of a twig occlusion is thought to be disruption of normal endothelium and laminar blood flow. Most pathology occurs at AV crossings, where thick, rigid-walled arteries compress the more flexible thin-walled vein neighbors. This compression or obstruction, or both, leads to disruption in normal laminar blood flow, leading to thrombus formation.3
Pathological processes such as arteriosclerosis that increase the thickness and rigidity of arterial walls are thought to be risk factors.4 Hypercoagulable conditions also increase the risk of thrombus formation and should be pursued in certain clinical presentations, where more typical risk factors are lacking.
In this patient with double AV crossings, development of a “twig” RVO provides circumstantial support for the presumed mechanism of injury. Given its stability and lack of other symptoms, we didn’t pursue further workup. A confounding point is that the patient continued treatment for her nAMD, and a lack of complications from the vein occlusion could be attributed to her ongoing anti-VEGF injections. RS
REFERENCES
1. Hayreh SS, Zimmerman B, McCarthy MJ, Podhajsky P. Systemic diseases associated with various types of retinal vein occlusion. Am J Ophthalmol. 2001;131:61-77.
2. Jaulim A, Ahmed B, Khanam T, Chatziralli IP. Branch retinal vein occlusion: Epidemiology, pathogenesis, risk factors, clinical features, diagnosis, and complications. An update of the literature. Retina. 2013;33:901-910.
3. Lee JY, Yoon YH, Kim HK, et al. Baseline characteristics and risk factors of retinal vein occlusion: A study by the Korean RVO Study Group. J Korean Med Sci. 2013;28:136-144.
4. Karia N. Retinal vein occlusion: Pathophysiology and treatment options. Clin Ophthalmol. 2010;4:809-816.



