
|
|
Bios Dr. Thomas is an associate in vitreoretinal surgery and uveitis at Tennessee Retina, with offices in central Tennessee and southern Kentucky. DISCLOSURE: Drs. Choi and Thomas have no relevant financial relationships to disclose. |
Management of noninfectious uveitis is an already daunting task for most of us. Doing so in the setting of pregnancy can cause even more apprehension as we have to consider avoiding harm not only to the mother but also to the fetus.
The course of noninfectious uveitis during pregnancy hasn’t been well established. However, general trends have been described. Numerous therapeutic options exist for controlling ocular inflammation in these patients. I’ll discuss what we know about the course of uveitis during pregnancy as well as management options and treatment approaches.
Autoimmune uveitis course
Pregnancy is known to have an ameliorating effect on a variety of autoimmune diseases. However, prospective, randomized control studies of pregnancy in uveitis have been lacking. Because uveitis is a rare condition, studies in pregnancy are limited by the number of eligible patients.
Nonetheless, a number of case reports and retrospective studies have described the course of uveitis in pregnancy.1–5 Based on these reports, uveitis appears to worsen in the first trimester, but tends to be less active later on. Subsequently, an increase in ocular inflammation occurs in the postpartum period.
A case series that included pregnant patients with Vogt-Koyanagi-Harada-associated uveitis, Behçet’s disease-associated uveitis and idiopathic uveitis reported an increased probability of having a uveitis flare in the first four months of pregnancy but a decreased probability later on.4 More than 50 percent of this cohort experienced a uveitic flare within six months of delivery.
An Australian study reported similar results.1 It included pregnant patients with uveitis associated with idiopathic disease, Behçet’s disease, sarcoidosis, Fuchs heterochromic uveitis, multifocal chorioretinitis, HLA-B27 disease and juvenile idiopathic arthritis. The study found a lower rate of flares in the second trimester vs. the first, but found no statistically significant difference in the rate of flares between the second and third trimesters. In the postpartum period, uveitis activity tended to relapse.
A retrospective cohort study compared pregnant and nonpregnant patients with uveitis, matching them for demographics and anatomical location of uveitis.3 Most of these patients had idiopathic uveitis, but other diseases were uveitis associated with relapsing polychondritis, Behçet’s disease, juvenile idiopathic arthritis, ankylosing spondylitis and inflammatory bowel disease. The flare rate was significantly lower in pregnant women than in nonpregnant periods or in nonpregnant controls. Among pregnant patients, uveitis flares were most common in the first trimester.
Based on the available studies, the consensus is that uveitis worsens during the first trimester of pregnancy, but then improves during the second and third trimesters. Increased rates of flare should be expected in the postpartum period.
Treatment approaches and options
Corticosteroids are the first line of treatment to acutely control ocular inflammation. The most common topical formulations used in uveitis are prednisolone and difluprednate (Durezol, Novartis). The periocular selection is triamcinolone, which is injected into the sub-Tenon’s space. Intravitreal choices include preservative-free triamcinolone, dexamethasone implant (Ozurdex, AbbVie) and long-acting fluocinolone acetonide implant (Yutiq, EyePoint Pharamaceuticals). Retisert (Bausch + Lomb), a surgically placed sustained-release fluocinolone implant, is another local option.
Short-acting local delivery options are the ideal initial approach to noninfectious uveitis during pregnancy because their systemic absorption is minimal, thus reducing the risk of potential harm to the mother and fetus.
Alternatives to local delivery
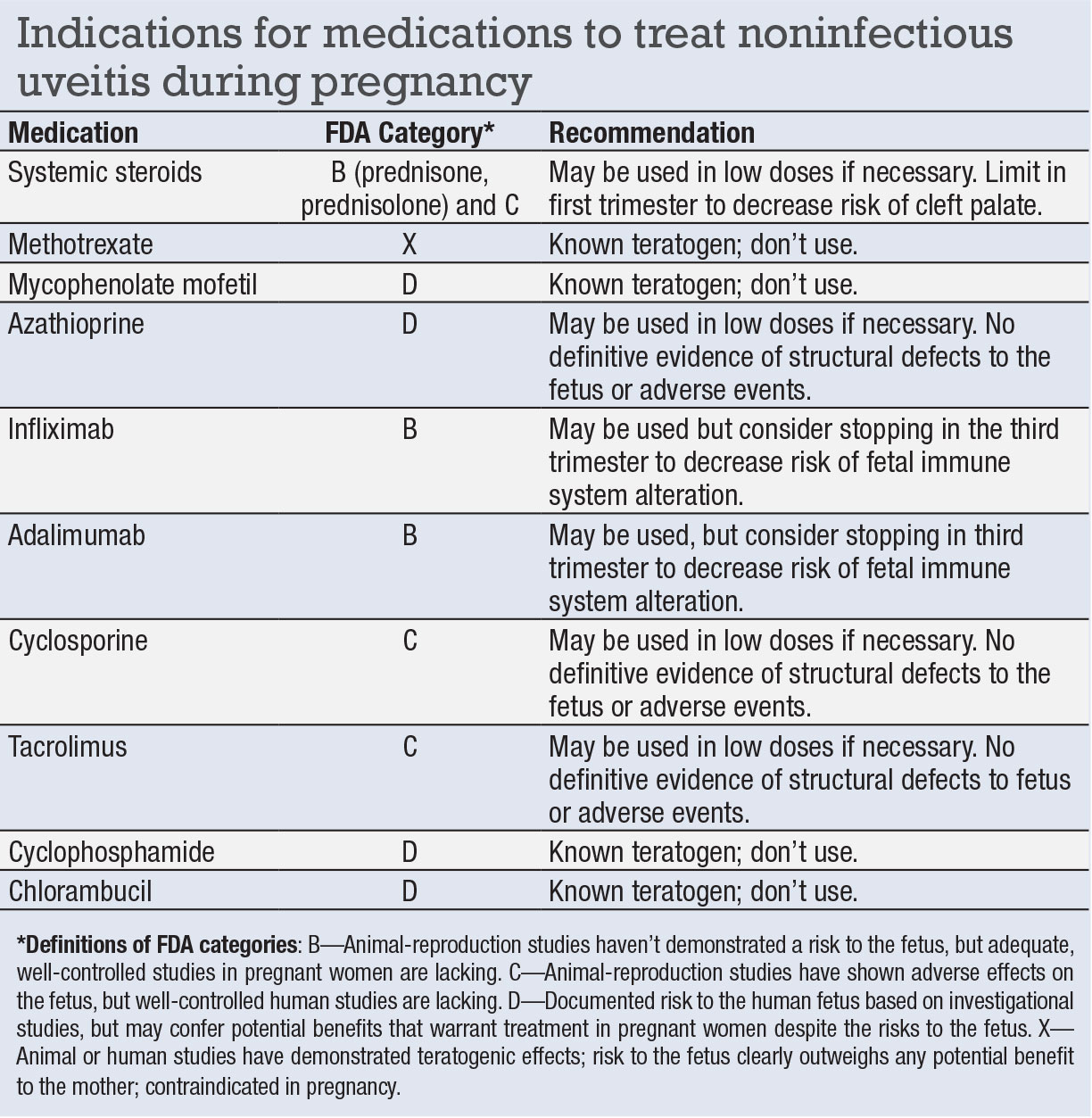
If local delivery results in inadequate control of ocular inflammation, then systemic corticosteroids are the next option. The FDA places most systemic corticosteroids in category C (adverse fetal effects reported in animal reproduction studies but well-controlled human studies are lacking). However, prednisone and prednisolone, the most commonly used systemic steroids in uveitis, are included in category B (animal reproduction studies have failed to demonstrate a risk to the fetus, and there are no adequate and well-controlled studies in pregnant women).
When using systemic steroids in the first trimester, discuss the potential risk of cleft lip and palate in infants.6,7 Systemic steroids can generally be used during pregnancy if medically warranted. However, they should be used in low doses and limit their use in the first trimester.
Immunomodulatory agents can be used for long-term control of noninfectious ocular inflammatory diseases. However, some of these medications are known teratogens and others lack sufficient safety data. Of the anti-metabolites, methotrexate and mycophenolate mofetil have been shown to be teratogenic and should be avoided.8–10
Azathioprine is an FDA category D medication (investigational studies have documented risk to the human fetus, but potential benefits may warrant treatment in pregnant women despite these risks). Azathioprine hasn’t been definitively shown to increase miscarriages or structural defects, but it should be used only in low doses for sight-threatening uveitis.10–13
The use of biologic response modifiers has grown rapidly in the treatment of uveitis. The most common are the tumor necrosis factor (TNF) inhibitors adalimumab (Humira, AbbVie) and infliximab (Remicade, Janssen Biotech), both of which are in FDA category B. No conclusive evidence has demonstrated either of them has antagonistic effects to the embryo or increase the risk of fetal death.14–16
Studies have shown the passage of TNF-inhibitors across the placenta appears to be the highest in the third trimester,17,18 leading to the recommendation that these agents should be stopped at the beginning of the third trimester to prevent potential immunosuppression in the infant.19
Certolizumab pegol (Cimzia, UCB) is a TNF-inhibitor that has minimal to no placental transfer from mother to fetus, and has been shown to be an effective option to control intraocular inflammation.20
The calcineurin inhibitors cyclosporine and tacrolimus have been used less frequently in uveitis since more efficacious medications such as TNF-inhibitors have emerged. The evidence is inconclusive that calcineurin inhibitors may increase the risk of prematurity and have unfavorable fetal side effects. They should only be used in pregnancy if medically warranted.21,22 Alkylating agents such as cyclophosphamide and chlorambucil are known teratogens and should be avoided during pregnancy.23–25
Bottom line
During pregnancy, ocular inflammation tends to increase in the first trimester and decrease in the second and third trimesters. An increase in uveitis flares should be expected postpartum. Local steroid delivery should be the first approach to controlling inflammation. Systemic steroids and immunomodulatory therapy may be used in cases refractory to local steroid administration. When choosing therapy, work closely with the obstetrician and patient to evaluate the risks and benefits for the mother and child. RS
REFERENCES
1. Chiam NP, Hall AJ, Stawell RJ, Busija L, Lim LL. The course of uveitis in pregnancy and postpartum. Br J Ophthalmol. 2013;97:1284-1288.
2. Chiam NP, Lim LL. Uveitis and gender: The course of uveitis in pregnancy. J Ophthalmol. 2014;2014:401915.
3. Kump LI, Cervantes-Castañeda RA, Androudi SN, Foster CS, Christen WG. Patterns of exacerbations of chronic non-infectious uveitis in pregnancy and puerperium. Ocul Immunol Inflamm. 2006;14:99-104.
4. Rabiah PK, Vitale AT. Noninfectious uveitis and pregnancy. Am J Ophthalmol. 2003;136:91-98.
5. Verhagen FH, Braakenburg AM, Kremer T, Drylewicz J, Rothova A, de Boer JH. Reduced number of relapses of human leucocyte antigen-B27-associated uveitis during pregnancy. Acta Ophthalmol. 2017;95:e798-e799.
6. Carmichael SL, Shaw GM. Maternal corticosteroid use and risk of selected congenital anomalies. Am J Med Genet. 1999;86:242-244.
7. Park-Wyllie L, Mazzotta P, Pastuszak A et al. Birth defects after maternal exposure to corticosteroids: Prospective cohort study and meta-analysis of epidemiological studies. Teratology. 2000;62:385-392.
8. Hoeltzenbein M, Elefant E, Vial T et al. Teratogenicity of mycophenolate confirmed in a prospective study of the European Network of Teratology Information Services. Am J Med Genet A. 2012;158A:588-596.
9. Perez-Aytes A, Ledo A, Boso V et al. In utero exposure to mycophenolate mofetil: A characteristic phenotype. Am J Med Genet A. 2008;146A:1-7.
10. Østensen M, Khamashta M, Lockshin M et al. Anti-inflammatory and immunosuppressive drugs and reproduction. Arthritis Res Ther. 2006;8:209.
11. Francella A, Dyan A, Bodian C, Rubin P, Chapman M, Present DH. The safety of 6-mercaptopurine for childbearing patients with inflammatory bowel disease: A retrospective cohort study. Gastroenterology. 2003;124:9-17.
12. Moskovitz DN, Bodian C, Chapman ML et al. The effect on the fetus of medications used to treat pregnant inflammatory bowel-disease patients. Am J Gastroenterol. 2004;99:656-661.
13. Polifka JE, Friedman JM. Teratogen update: Azathioprine and 6-mercaptopurine. Teratology. 2002;65:240-261.
14. Chambers CD, Johnson DL, Xu R et al. Birth outcomes in women who have taken adalimumab in pregnancy: A prospective cohort study. PLoS One. 2019;14:e0223603.
15. Roux CH, Brocq O, Breuil V, Albert C, Euller-Ziegler L. Pregnancy in rheumatology patients exposed to anti-tumour necrosis factor (TNF)-alpha therapy. Rheumatology (Oxford). 2007;46:695-698.
16. Vinet E, Pineau C, Gordon C, Clarke AE, Bernatsky S. Anti-TNF therapy and pregnancy outcomes in women with inflammatory arthritis. Expert Rev Clin Immunol. 2009;5:27-34.
17. Mahadevan U, Wolf DC, Dubinsky M et al. Placental transfer of anti-tumor necrosis factor agents in pregnant patients with inflammatory bowel disease. Clin Gastroenterol Hepatol. 2013;11:286-292.
18. Zelinkova Z, de Haar C, de Ridder L et al. High intra-uterine exposure to infliximab following maternal anti-TNF treatment during pregnancy. Aliment Pharmacol Ther. 2011;33:1053-1058.
19. O’Donnell S, O’Morain C. Review article: Use of antitumour necrosis factor therapy in inflammatory bowel disease during pregnancy and conception. Aliment Pharmacol Ther. 2008;27:885-894.
20. Prieto-Peña D, Calderón-Goercke M, Adán A et al. Efficacy and safety of certolizumab pegol in pregnant women with uveitis. Recommendations on the management with immunosuppressive and biologic therapies in uveitis during pregnancy. Clin Exp Rheumatol. Published online October 9, 2020.
21. Bar Oz B, Hackman R, Einarson T, Koren G. Pregnancy outcome after cyclosporine therapy during pregnancy: A meta-analysis. Transplantation. 2001;71:1051-1055.
22. Jain AB, Reyes J, Marcos A et al. Pregnancy after liver transplantation with tacrolimus immunosuppression: A single center’s experience update at 13 years. Transplantation. 2003;76:827-832.
23. Botta JA, Hawkins HC, Weikel JH. Effects of cyclophosphamide on fertility and general reproductive performance of rats. Toxicol Appl Pharmacol. 1974;27:602-611.
24. Enns GM, Roeder E, Chan RT, Ali-Khan Catts Z, Cox VA, Golabi M. Apparent cyclophosphamide (cytoxan) embryopathy: A distinct phenotype. Am J Med Genet. 1999;86:237-241.
25. Steege JF, Caldwell DS. Renal agenesis after first trimester exposure to chlorambucil. South Med J. 1980;73:1414-1415.




