 |
|
Bios Dr. Grewal is an associate professor of ophthalmology at Duke University. |
Acute retinal necrosis (ARN), first described in 1971,1 is a progressive inflammatory syndrome now defined by the American Uveitis Society on the basis of clinical appearance of (1) one or more foci of retinal necrosis located in the peripheral retina with distinct borders, (2) rapid progression with no antiviral therapy, (3) circumferential spread, (4) evidence of occlusive vasculopathy with arterial involvement and (5) significant inflammatory reaction in the vitreous and anterior chamber.2
ARN usually results from viral infections, primarily caused by the varicella-zoster virus (VZV), herpes simplex virus type 1 and 2 (HSV-1 and HSV-2) and less commonly cytomegalovirus (CMV). Toxoplasma chorioretinitis and syphilitic retinitis can also mimic retinal necrosis, among other manifestations, and are included in the differential and workup.
Presenting symptoms often include redness, photophobia, floaters and blurred vision.
On fundus examination there are multifocal areas of whitening in the peripheral retina corresponding to active retinal necrosis, which may become confluent and circumferentially progress to involve the posterior pole without prompt treatment. Vascular sheathing, perivascular hemorrhages, vascular occlusion and obliteration of arterioles may be present. Dense vitreous inflammation may develop as the disease progresses. Retinal breaks often develop within the peripheral necrotic retinal lesions. It’s helpful to capture fundus photographs at presentation in order to monitor response to treatment. Features on OCT can sometimes be helpful to distinguish herpetic retinitis from toxoplasma chorioretinitis where there’s often choroidal thickening and from syphilitic retinitis where there’s typically involvement of the outer retina.3 Fluorescein angiography may demonstrate signs of occlusive arteritis and areas of ischemia.
In immunodeficient patients, a variant—progressive outer retinal necrosis syndrome—can occur, most often caused by VZV and with early posterior pole involvement, absence of vitreous reactivity and relative sparing of the retinal vessels.4
 |
|
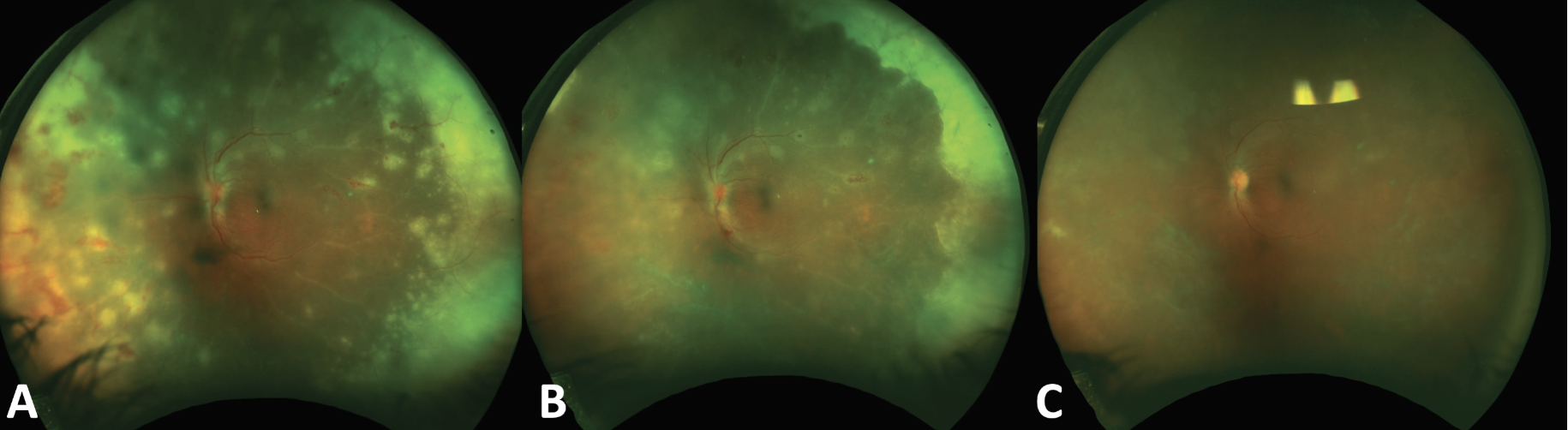
A 45-year old immunocompetent male with acute retinal necrosis secondary to varicella zoster virus (VZV). On presentation (A), there were multiple near-confluent areas of whitening in the peripheral retina corresponding to active retinal necrosis, vasculitis, perivascular hemorrhages and vascular sheathing. Three days after initiation of combined oral and intravitreal treatment there was significant consolidation of the retinitis (B) and by two weeks (C) the retinitis was completely consolidated and vasculitis had improved following the initiation of oral steroids to control the inflammatory component. |
Diagnosis
Polymerase chain reaction testing of ocular samples for HSV and VZV has a very high sensitivity and aqueous and vitreous sampling have similar yields.5 It’s important that immediate treatment for ARN should be initiated without waiting for the PCR results due to the risk of rapid progression. Additional infectious etiologies including human immunodeficiency virus, tuberculosis and syphilis should be tested for. Serum testing for herpesvirus antibodies doesn’t add any value in the diagnosis of ARN.
Epstein-Barr Virus (EBV) as an etiologic agent for ARN hasn’t yet been confirmed in retinal or vitreous specimens.
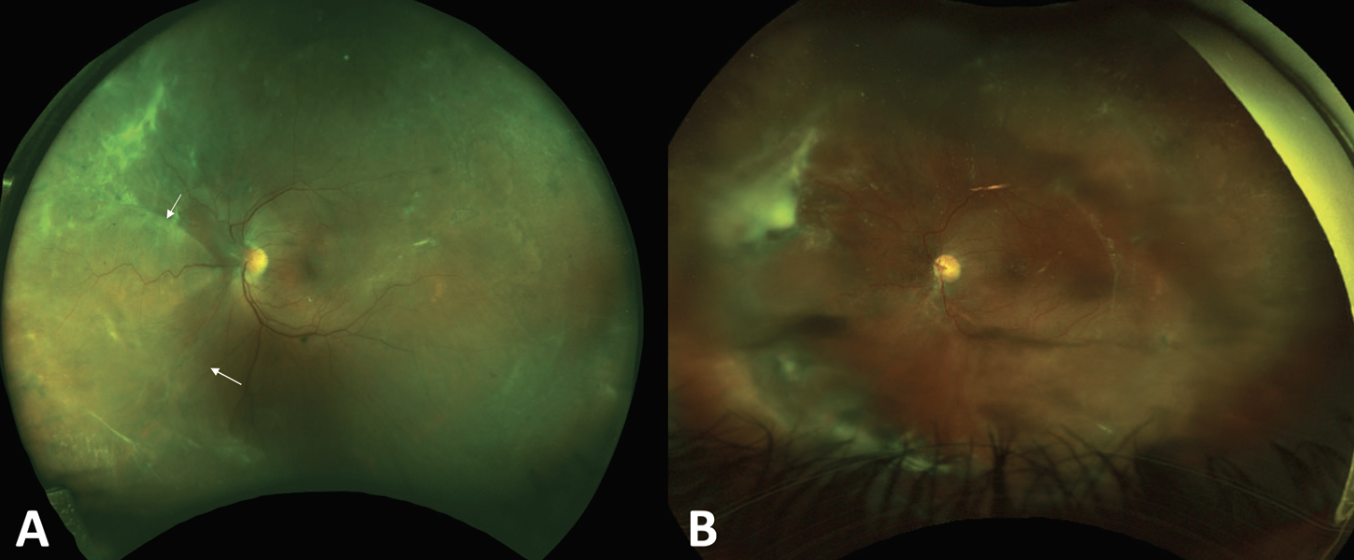 |
| Two months later, the same patient from Figure 1 developed a nasal rhegmatogenous retinal detachment (A) requiring surgery with a scleral buckle and pars plana vitrectomy with silicone oil for retinal re-attachment (B). |
Antiviral Treatment
Early administration of antivirals is critical in the treatment of ARN. This includes intravenous acyclovir or oral valacyclovir. Oral and intravenous therapy have comparable outcomes and either is effective for induction therapy. Oral antiviral medications with greater bioavailability (valacyclovir, famciclovir) and increased use of adjunct intravitreal antivirals have led to more frequently initiating treatment with oral antivirals. This treatment algorithm can avoid the need for a hospital admission and intravenous medication.6 There’s level II and III evidence suggesting that the combination of intravitreal and systemic antiviral therapy may have greater therapeutic efficacy than systemic therapy alone.5,7
Intravitreal monotherapy is inadequate as involvement of the fellow eye has been reported in up to 70 percent of patients without systemic treatment. Combination of systemic and intravitreal treatment is typically used in more severe or progressive cases, immunodeficient individuals or in those with more posterior zone involvement of the retinitis.
Intravitreal foscarnet (2.4 mg/0.1 mL) or ganciclovir (2 mg/0.1 mL) may be given as adjuvant intravitreal therapy. There’s animal data to suggest that intravitreal ganciclovir has better retinal pharmacokinetics than intravitreal foscarnet.8 Ganciclovir concentration remains at a higher therapeutic level than foscarnet for 72 hours post injection and its concentration in the retina remains higher. Ganciclovir has activity against CMV and HSV, and can be administered intravenously, orally or intravitreally.
Valacyclovir is an orally administered prodrug that’s converted to acyclovir and has a much higher bioavailability vs. oral acyclovir. Oral valacyclovir (2 g three times a day) can reach concentrations in the vitreous in the inhibitory ranges for HSV-1, HSV-2 and VZV,9 similar to intravenous acyclovir 10 mg/kg t.i.d. Following initiation of therapy, there can still be progression of retinitis, particularly in the first 24 hours before consolidation of retinitis starts to occur. The duration of induction therapy should be titrated based on response of retinitis and it may take longer than two to three weeks before the retinitis adequately consolidates.
Acyclovir and valacyclovir are well tolerated in the oral form but intravenously administered can cause neurotoxicity and renal toxicity.10 Ganciclovir, when given intravenously, may cause bone marrow suppression, anemia, granulocytopenia and thrombocytopenia and renal toxicity.10 Renal function should be tested prior to initiation of antiviral therapy to monitor for drug toxicity and subsequent dose adjustments, particularly those with impaired renal function.
Depending on the severity of inflammation, topical and systemic corticosteroids are frequently administered alongside antivirals. While topical steroids can be initiated immediately, oral steroids are added 24 to 48 hours after observing the initial response to antiviral treatment. Avoid local steroids.
Resistance to Therapy
Acyclovir-resistant HSV has been reported to be caused by a mutation of the thymidine kinase gene A156V.11 Acyclovir-resistant HSV almost triples the time to achieve a viral load reduction with acyclovir treatment12 and in such cases, famciclovir can be considered. CMV resistance can occur due to mutations in the viral UL97 kinase and UL54 DNA polymerase genes. Resistance can sometimes be overcome by intravitreal dosing. Letermovir is a novel antiviral recently approved for CMV prophylaxis following hematopoietic cell transplantation and has been shown to be effective as salvage treatment for ganciclovir-resistant CMV retinitis.13
Role of Long Term Antiviral Prophylaxis
Herpes viruses can remain latent for a lifetime and may undergo episodic reactivation triggered by various stimuli, including immunosuppression. Contralateral ocular involvement or encephalitis can occur, anywhere from within a few months to several years later, underscoring the importance of long term antiviral prophylaxis, often for a lifetime in immunosuppressed individuals.14
Visual Outcomes
Visual outcomes are limited due to complications such retinal detachment, chronic vitreous inflammation, epiretinal membrane, macular ischemia, macular edema and optic neuropathy.15
Nearly half of affected eyes have a visual acuity of ≤20/200, six months after the onset of ARN.
The role of prophylactic laser and/or pars plana vitrectomy to reduce risk of retinal detachment in ARN remains unclear. The rationale behind vitrectomy is to remove inflammatory mediators, clear the media and perform endolaser. A recent meta-analysis found that prophylactic pars plana vitrectomy could reduce the risk of retinal detachment compared to antiviral treatment alone, although the final vision of the vitrectomy group was worse.16 Vitrectomy in younger patients with ARN is particularly challenging due to the difficulty in removing the posterior hyaloid and risk of causing breaks over the thin necrotic retina.
In conclusion, ARN, while rare, can result in severe ocular morbidity if not accurately diagnosed and immediately treated. It’s important to manage the concurrent inflammatory response, and long-term prophylaxis therapy—potentially for life—is critical to reduce the risk of fellow-eye involvement. RS
REFERENCES
1. Urayama A. Unilateral acute uveitis with periarteritis and detachment. Jpn J Clin Ophthalmol 1971;25:607-19.
2. Holland GN. Standard diagnostic criteria for the acute retinal necrosis syndrome. American Journal of Ophthalmology 1994;117:5:663-66.
3. Agarwal A, Invernizzi A. The role of optical coherence tomography and optical coherence tomography angiography in the differential diagnosis of posterior uveitis. Ocul Immunol Inflamm 2022;30:3:682-89.
4. Forster DJ, Dugel PU, Frangieh GT, Liggett PE, Rao NA. Rapidly progressive outer retinal necrosis in the acquired immunodeficiency syndrome. Am J Ophthalmol 1990;110:4:341.
5. Schoenberger SD, Kim SJ, Thorne JE, et al. Diagnosis and treatment of acute retinal necrosis: A report by the American Academy of Ophthalmology. Ophthalmology 2017;124:3:382-92.
6. Aizman A, Johnson MW, Elner SG. Treatment of acute retinal necrosis syndrome with oral antiviral medications. Ophthalmology 2007;114:2:307-12.
7. Putera I, Ridwan AS, Dewi M, et al. Antiviral treatment for acute retinal necrosis: A systematic review and meta-analysis. Surv Ophthalmol 2024;69:1:67-84 doi: 10.1016/j.survophthal.2023.09.004 published Online First: 20230927.
8. Lopez-Cortes LF, Pastor-Ramos MT, Ruiz-Valderas R, et al. Physiology and pharmacology-Intravitreal pharmacokinetics and concentrations of ganciclovir and foscarnet after intravitreal administration in rabbits. Investigative Ophthalmology and Visual Science 2001;42:5:1024-28.
9. Huynh TH, Johnson MW, Comer GM, Fish DN. Vitreous penetration of orally administered valacyclovir. American Journal of Ophthalmology 2008;145:4:682-86.
10. Tam PM, Hooper CY, Lightman S. Antiviral selection in the management of acute retinal necrosis. Clinical Ophthalmology 2010:11-20.
11. Bergmann M, Beer R, Kofler M, Helbok R, Pfausler B, Schmutzhard E. Acyclovir resistance in herpes simplex virus type I encephalitis: A case report. Journal of Neurovirology 2017;23:335-37.
12. Hafidi M, Janin-Manificat H, Denis P, et al. Acute retinal necrosis: Virological features using quantitative polymerase chain reaction, therapeutic management, and clinical outcomes. American Journal of Ophthalmology 2019;208:376-86.
13. Turner N, Strand A, Grewal DS, et al. Use of letermovir as salvage therapy for drug-resistant cytomegalovirus retinitis. Antimicrob Agents Chemother 2019;63:3.
14. Palay DA, Sternberg Jr P, Davis J, et al. Decrease in the risk of bilateral acute retinal necrosis by acyclovir therapy. American Journal of Ophthalmology 1991;112:3:250-55.
15. Wong RW, Jumper JM, McDonald HR, et al. Emerging concepts in the management of acute retinal necrosis. Br J Ophthalmol 2013;97:5:545-52.
16. Fan S, Lin D, Wang Y. Role of prophylactic vitrectomy in acute retinal necrosis in preventing rhegmatogenous retinal detachment: Systematic review and meta-analysis. Ocular Immunology and Inflammation 2022;30:2:515-19.




