 |
|
Bios Dr. Mosenia is an Dr. Crowell is an assistant professor of ophthalmology at Dell Medical School, clinical director of UT Health Austin’s Mitchel and Shannon Wong Eye Institute and the Ascension Seton Network ophthalmology specialty service chief for Texas. Dr. Thomas is a partner in vitreoretinal surgery and uveitis at Tennessee Retina. DISCLOSURES: Dr. Mosenia and Dr. Crowell have no relevant disclosures. Dr. Thomas is a consultant to Allergan/AbbVie, Alimera Sciences, Avesis, EyePoint Pharmaceuticals, Genentech/Roche and Novartis. |
Birdshot chorioretinopathy is a chronic, bilateral, posterior uveitis characterized by multifocal, cream-colored choroidal lesions in the posterior pole and mid-periphery at the level of retinal pigment epithelium or deeper layers.1,2
Birdshot chorioretinopathy (BSCR) is a T-cell mediated autoimmune disorder that’s strongly associated with human leukocyte antigen (HLA) A29. More than 85 to 95 percent of affected patients test positive for this allele.3–5 BSCR primarily affects individuals of northern European descent ages 40 to 60.6 years.
Patients typically present with floaters, flashes, loss of peripheral vision and nyctalopia. However, the most common chief complaint is decreased visual acuity.6 Diagnosis can be delayed significantly because BSCR has vague symptomatology and an insidious early course with stable vision loss despite significant tissue loss. 7
Diagnosis and monitoring
While BSCR is diagnosed clinically, examination findings, diagnostic testing and multimodal imaging all can play an important role in identifying patients who require further evaluation and monitoring of treatment.
Clinical findings
On exam, you can expect to find mild to no anterior chamber inflammation and no to moderate vitritis.8 Retinal vascular leakage may be the only noticeable finding early on, while the characteristic multifocal, cream-colored or yellow-orange, oval or round lesions emerge from around the optic nerve later in the disease course (Figure 1).
Cystoid macular edema, vascular attenuation, subretinal neovascularization and optic nerve atrophy may accompany advance-stage BSCR.9 Vision loss is more notable in later stages and correlates highly with macular edema or photoreceptor loss secondary to diffuse retinal damage.9,10
Visual acuity and fields
Studies have demonstrated a robust correlation between VA at disease onset and long-term visual prognosis, emphasizing the importance of prompt diagnosis and treatment of BSCR.9
Besides the symptoms described, patients can experience significant visual field defects despite well-preserved central VA, including diffuse constriction, paracentral scotomas and blind spot enlargement.11
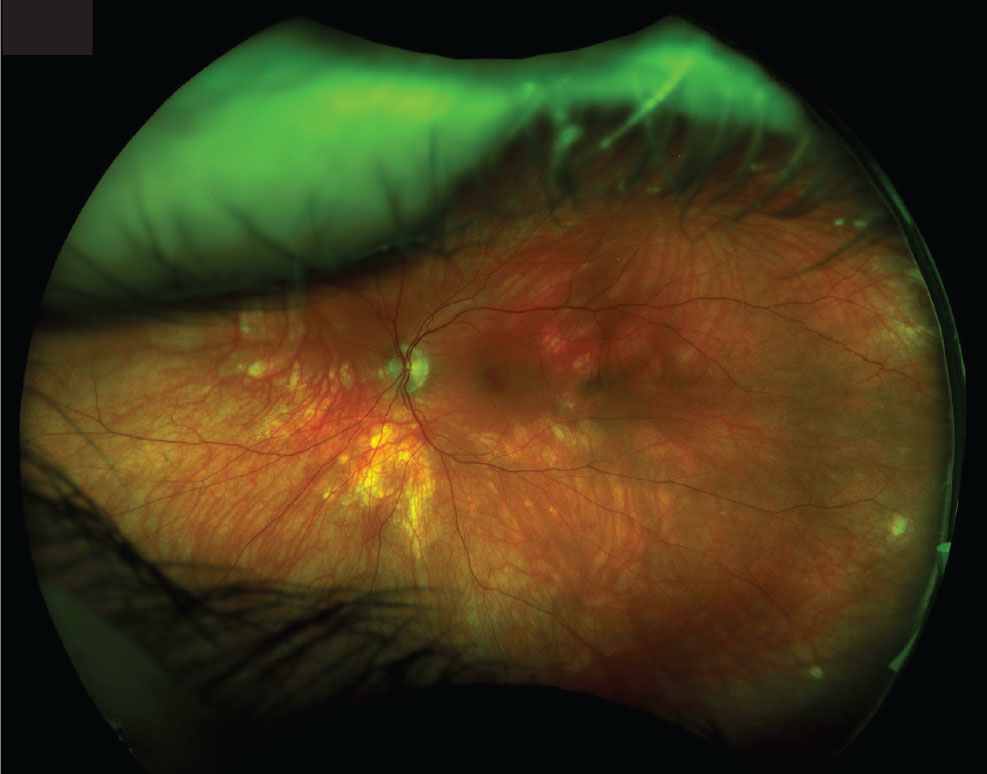 |
|
Figure 1. Fundus examination reveals creamy hypopigmented ovoid lesions in the peripapillary and midperipheral region. |
Visual electrodiagnostic testing
Electrodiagnostic testing is one of the oldest diagnostic modalities of BSCR. In earlier stages of BSCR, testing may reveal a disproportionate decrease in B-wave amplitude relative to A-wave amplitude.
As the disease progresses, changes to the inner retinal function of the cone and rod systems present with a delayed cone mediated 30 Hz flicker on electroretinograms, which is the most sensitive electrodiagnostic test for assessing and monitoring BSCR.12 Nevertheless, electrodiagnostic testing is time-consuming and isn’t readily available in most clinics.
Multimodal imaging
Small birdshot lesions represent areas of focal inflammation and cause abnormalities on various types of imaging, which can be monitored over time. Fluorescein angiography is the gold-standard imaging study for evaluating vascular abnormalities, including vascular leakage or hypoperfusion (Figure 2).13 Additionally, FA may reveal macular edema and hyperfluorescent choroidal lesions.
Indocyanine green angiography is more sensitive than FA and fundoscopy in revealing choroidal lesions (Figure 3), especially early in the disease course.14 Optical coherence tomography provides a less-invasive alternative for monitoring BSCR.
In addition to revealing the choroidal lesions and macular edema, it can be used to quantify retinal thinning and loss of the third highly reflective band, both of which have been associated with worse visual outcomes.13,15
The highly reflective band is a linear band found at the photoreceptor level. OCT angiography can identify abnormal flow signals, similar to those seen on ICGA.16 Hyporeflectivity in the macular and peripapillary regions on enhanced-depth OCT, which enables the analysis of deeper choroidal levels, is another common finding in BSCR; it has been reported in up to 64 percent of patients.17
More recently, retina specialists have used FA to reveal important information about retinal health in BSCR. Particularly, peripapillary confluent hypoautofluorescence is shown to be present in 73 percent of eyes and is associated with chronicity and severity of BSCR.18 Authors have also reported linear hypoautofluorescence streaks along the retinal vessels and an arcuate pattern of hypoautofluorescence at the posterior pole.18,19
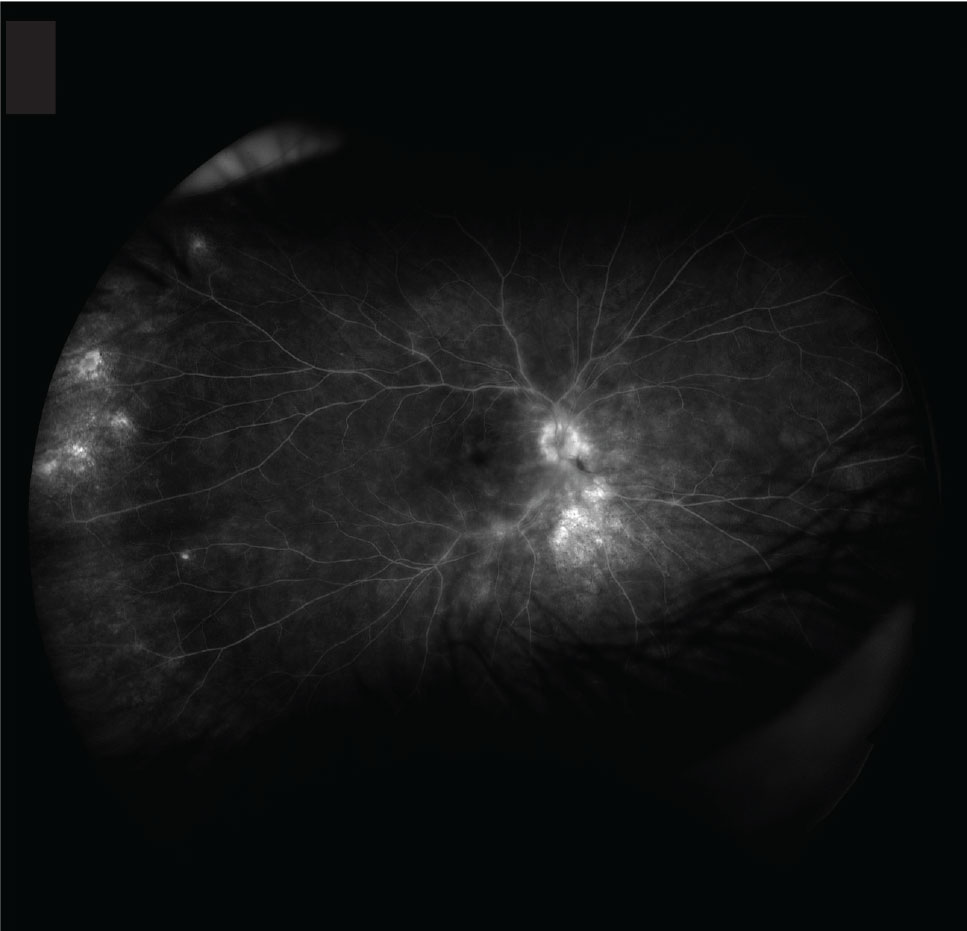 |
|
Figure 2. Fluorescein angiography demonstrates leakage of the retinal vessels and optic disc, along with angiographic macular edema. |
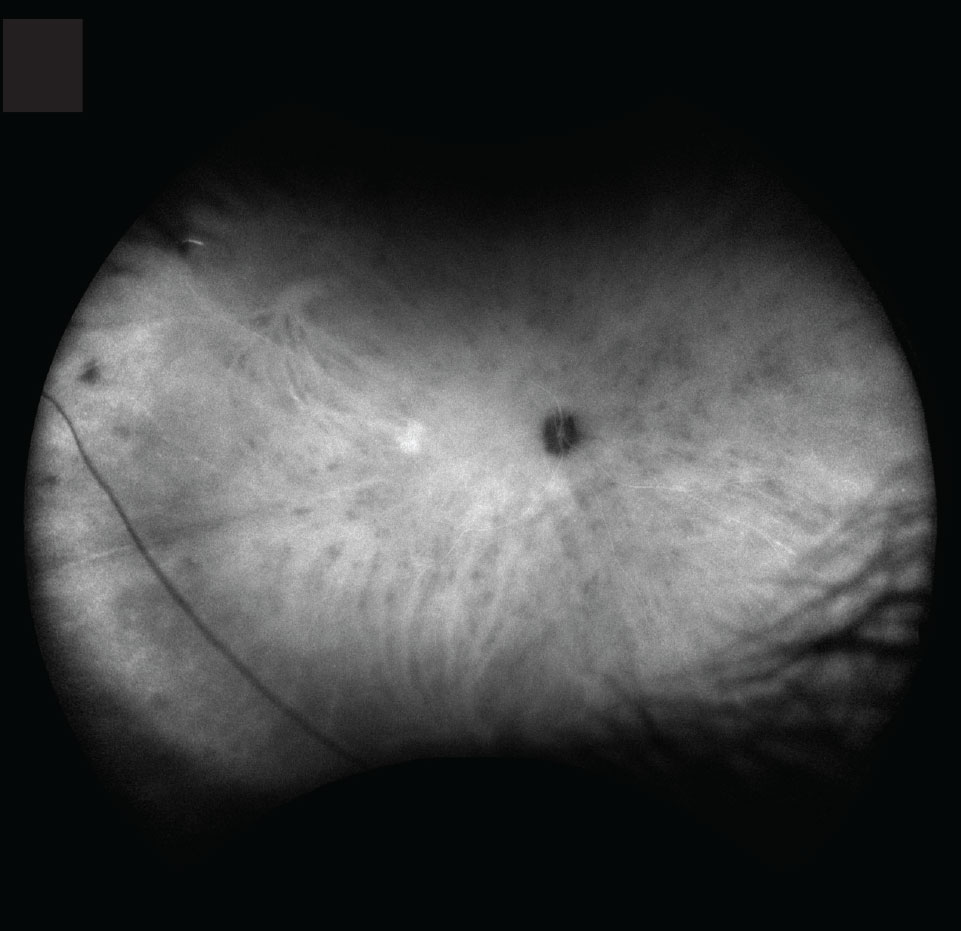 |
|
Figure 3. Indocyanine green angiography demonstrates hypofluorescent lesions throughout the choroid, some corresponding to the lesions seen on color fundus photography. |
Monitoring treatment
Imaging and diagnostic tools are also valuable in the treatment and monitoring of BSCR. They facilitate treatment decisions, such as determining the need to escalate or de-escalate care.
Corticosteroids are the mainstay initial treatment in BSCR. Immunosuppressive therapy, in combination with steroids, has advantages in preserving visual function and reducing the adverse effects associated with high-dose corticosteroids compared with corticosteroid therapy alone.20
Calcineurin inhibitors, which inhibit T-cell signaling, and antimetabolites are typically the first immunosuppressive treatments clinicians consider. Mycophenolate mofetil has been shown to be more effective compared to other antimetabolites in treating posterior uveitis and panuveitis.21 However, a randomized clinical trial of methotrexate and mycophenolate mofetil revealed statistically insignificant higher treatment success in methotrexate for posterior and panuveitis in general.22
 |
Stepping up to biologics
In most patients who respond to concomitant immunosuppressive therapy, corticosteroids can be successfully tapered and possibly discontinued.23 However, if patients don’t respond to conventional immunosuppressive therapies, then stepping up to biologics is indicated.
Tumor necrosis factor (TNF)-alpha inhibitors, such as infliximab (Remicade, Janssen) and adalimumab (Humira, AbbVie), have been reported to be effective in treating some forms of refractory uveitis, including BSCR.24–26
Patients treated with adalimumab demonstrated VA improvement and had successful tapering of concomitant immunosuppressive therapy. However, complete disease remission with adalimumab alone can be challenging.26 Some authors have reported successful use of other classes of biologics, but current evidence for their use in BSCR is limited.27
Localized steroid administration
Local steroid injections are often indicted in patients who have persistent macular edema despite systemic therapy. In patients who can’t tolerate or don’t respond adequately to systemic therapy, local therapy with intravitreal corticosteroid implants can be a viable option. The intravitreal 0.59 mg fluocinolone implant (Retisert, Bausch + Lomb) is a well-described treatment for uveitis. It exerts its effect locally and may spare the side effects of systemic immunosuppression.
Based on the Multicenter Uveitis Steroid Treatment (MUST) trial, patients with uveitis who receive systemic therapy and intravitreal implants have similar visual outcomes for up to 4.5 years of treatment.28 However, after seven years, systemic therapy was associated with better visual outcomes.29
In BSCR particularly, steroid implants have been associated with an intraocular pressure response more robust than seen in patients with other types of uveitis. Up to 40 percent of patients can need filtering surgery, and all of them will need to undergo cataract surgery.30,31
Long-term treatment and outcomes
Treatment success with immunosuppression after one year of therapy has been reported at 67 to 90 percent.23 Single- or dual-agent immunosuppression has been successfully used to control BSCR while sparing steroid use to minimize systemic side effects. Steroid sparing, defined as successfully tapering the prednisone dose to ≤ 7.5 mg, is generally considered safe for long-term use and can be achieved within six months.
One recent study suggested that up to 90 percent of patients with BSCR can achieve steroid sparing, and as many as 75 percent can completely discontinue steroid use.23 However, more than 40 percent of patients can have reactivation of BSCR during tapering, requiring the use of a second immunosuppressive therapy.
Once steroid tapering happens, it may be necessary to continue treatment with higher-dose immunosuppressive therapy for at least two years before tapering the immunosuppressive agents to decrease the risk of relapse after remission.23
Bottom line
Diagnosing BSCR can be challenging because of its gradual onset and ambiguous symptoms. Several diagnostic methods and imaging techniques exist for the treatment of BSCR, each with its own set of benefits and drawbacks at different stages of the illness.
Treatment involves a combination of oral steroids and immunosuppressive therapy to address inflammation, with most patients successfully reducing steroid use over time. Effective management of BSCR requires ongoing monitoring, even after the patient has successfully stopped immunosuppression therapy. RS
REFERENCES
1. Ryan SJ, Maumenee AE. Birdshot retinochoroidopathy. Am J Ophthalmol. 1980;89:31-45.
2. Kaplan HJ, Aaberg TM. Birdshot retinochoroidopathy. Am J Ophthalmol. 1980;90:773-782.
3. Priem HA, Kijlstra A, Noens L, Baarsma GS, De Laey JJ, Oosterhuis JA. Am J Ophthalmol. 1988;105:182-185.
4. Kuiper JJW, Rothova A, Schellekens PAW, Ossewaarde-van Norel A, Bloem AC, Mutis T. Detection of choroid- and retina-antigen reactive CD8+ and CD4+ T lymphocytes in the vitreous fluid of patients with birdshot chorioretinopathy. Hum Immunol. 2014;75:570-577.
5. Nussenblatt RB, Mittal KK, Ryan S, Green WR, Maumenee AE. Birdshot retinochoroidopathy associated with Hla-A29 antigen and immune responsiveness to retinal S-antigen. Am J Ophthalmol. 1982;94:147-158.
6. Monnet D, Brézin AP, Holland GN, et al. Longitudinal cohort study of patients with birdshot chorioretinopathy. I. Baseline clinical characteristics. Am J Ophthalmol. 2006;141:135-142.
7. Taylor S, Menezo V. Birdshot uveitis: current and emerging treatment options. Clin Ophthalmol. Published online December 2013:73.73.81.
8. Standardization of Uveitis Nomenclature (SUN) Working Group. Classification criteria for birdshot chorioretinitis. Am J Ophthalmol. 2021;228:65-71.
9. Rothova A. Birdshot chorioretinopathy: Long-term manifestations and visual prognosis. Evid-Based Eye Care. 2004;5:204-205.
10. Thorne JE, Jabs DA, Peters GB, Hair D, Dunn JP, Kempen JH. Birdshot retinochoroidopathy: Ocular complications and visual impairment. Am J Ophthalmol. 2005;140:45.e1-45.e8.
11. Gasch AT, Smith JA, Whitcup SM. Birdshot retinochoroidopathy. Br J Ophthalmol. 1999;83:241-249.
12. Sobrin L, Lam BL, Liu M, Feuer WJ, Davis JL. Electroretinographic monitoring in birdshot chorioretinopathy. Am J Ophthalmol. 2005;140:52.e1-52.e18.
13. Monnet D, Levinson RD, Holland GN, Haddad L, Yu F, Brézin AP. Longitudinal cohort study of patients with birdshot chorioretinopathy. III. Macular imaging at baseline. Am J Ophthalmol. 2007;144:818-828.
14. Fardeau C, Herbort CP, Kullmann N, Quentel G, LeHoang P. Indocyanine green angiography in birdshot chorioretinopathy. Ophthalmology. 1999;106:1928-1934.
15. Shields CL, Materin MA, Walker C, Marr BP, Shields JA. Photoreceptor loss overlying congenital hypertrophy of the retinal pigment epithelium by optical coherence tomography. Ophthalmology. 2006;113:661-665.
16. Pepple KL, Chu Z, Weinstein J, Munk MR, Van Gelder RN, Wang RK. Use of en face swept-source optical coherence tomography angiography in identifying choroidal flow voids in 3 patients with birdshot chorioretinopathy. JAMA Ophthalmol. 2018;136:1288-1292.
17. Böni C, Thorne JE, Spaide RF, et al. Choroidal findings in eyes with birdshot chorioretinitis using enhanced-depth optical coherence tomography. Invest Opthalmol Vis Sci. 2016;57:OCT591-599.
18. Böni C, Thorne JE, Spaide RF, et al. Fundus autofluorescence findings in eyes with birdshot chorioretinitis. Invest Opthalmol Vis Sci. 2017;58:4015-4025.
19. Koizumi H, Pozzoni MC, Spaide RF. Fundus autofluorescence in birdshot chorioretinopathy. Ophthalmology. 2008;115:e15-e20.
20. Kiss S, Ahmed M, Letko E, Foster C. Long-term follow-up of patients with birdshot retinochoroidopathy treated with corticosteroid-sparing systemic immunomodulatory therapy. Ophthalmology. 2005;112:1066-1071.e2.
21. Galor A, Jabs DA, Leder HA, et al. Comparison of antimetabolite drugs as corticosteroid-sparing therapy for noninfectious ocular inflammation. Ophthalmology. 2008;115:1826-1832.
22. Rathinam SR, Babu M, Thundikandy R, et al. A randomized clinical trial comparing methotrexate and mycophenolate mofetil for noninfectious uveitis. Ophthalmology. 2014;121:1863-1870.
23. Crowell EL, France R, Majmudar P, Jabs DA, Thorne JE. Treatment outcomes in birdshot chorioretinitis. Ophthalmol Retina. 2022;6:620-627.
24. Artornsombudh P, Gevorgyan O, Payal A, Siddique SS, Foster CS. Infliximab treatment of patients with birdshot retinochoroidopathy. Ophthalmology. 2013;120:588-592.
25. Suhler EB. A prospective trial of infliximab therapy for refractory uveitis: Preliminary safety and efficacy outcomes. Arch Ophthalmol. 2005;123:903-912.
26. Huis I Het Veld PI, van Asten F, Kuijpers RWAM, Rothova A, de Jong EK, Hoyng CB. Adalimumab therapy for refractory birdshot chorioretinopathy. Retina. 2019;39:2189-2197.
27. Muselier A, Bielefeld P, Bidot S, Vinit J, Besancenot JF, Bron A. Efficacy of tocilizumab in two patients with anti-TNF-alpha refractory uveitis. Ocul Immunol Inflamm. 2011;19:382-383.
28. Multicenter Uveitis Steroid Treatment (MUST) Trial Research Group; Kempen JH, Altawell MM, Drye LT, et al. Benefits of systemic anti-inflammatory therapy versus fluocinolone acetonide intraocular implant for intermediate uveitis, posterior uveitis, and panuveitis. Ophthalmology. 2015;122:1967-1975.
29. Writing Committee for the Multicenter Uveitis Steroid Treatment (MUST) Trial and Follow-up Study Research Group, Kempen JH, Altaweel MM, et al. Association between long-lasting intravitreous fluocinolone acetonide implant vs systemic anti-inflammatory therapy and visual acuity at 7 years among patients with intermediate, posterior, or panuveitis. JAMA. 2017;317:1993-2005.
30. Burkholder BM, Wang J, Dunn JP, Nguyen QD, Thorne JE. Postoperative outcomes after fluocinolone acetonide implant surgery in patients with birdshot chorioretinitis and other types of posterior and panuveitis. Retina. 2013;33:1684-1693.
31. Jaffe GJ, Martin D, Callanan D, Pearson PA, Levy B, Comstock T. Fluocinolone acetonide implant (Retisert) for noninfectious posterior uveitis. Ophthalmology. 2006;113:1020-1027.




