Take-home points
|
 |
|
Bios The authors are with the department of medicine-ophthalmology at the University of Udine in Udine, Italy. Dr. Sarao and Dr. Lanzetta are also with the Istituto Europeo di Microchirurgia Oculare (European Institute of Ocular Microsurgery) in Udine. DISCLOSURES: Dr. Sarao is a consultant to I-Care. Dr. Lanzetta is a consultant to AbbVie, Aerie, Apellis Pharmaceuticals, Bausch + Lomb, Bayer, Biogen, Boehringer Ingelheim, Genentech, I-Care, Novartis, Ocular Therapeutix, Outlook Therapeutics and Roche. Dr. Martinuzzi has no relevant financial disclosures. |
As we all know, age-related macular degeneration is the primary cause of blindness worldwide.1 It’s a progressive condition that affects the macula, resulting in damage to the retina, retinal pigment epithelium and choriocapillaris, ultimately leading to central vision impairment.1,2 This maculopathy affects a significant proportion of adults and it can cause severe and permanent visual impairment, and even legal blindness, presenting a considerable public health issue.3–5
AMD is categorized into early, intermediate and late stages.2 The late stage encompasses two primary forms that aren’t mutually exclusive: geographic atrophy and neovascular AMD.1,6 GA is characterized by localized sharply demarcated atrophy of outer retinal tissue, RPE and choriocapillaris.7 The neovascular form is associated with choroidal neovascularization, which leads to intraretinal or subretinal leakage, hemorrhage and RPE detachment.1,6
Is AMD categorization outdated?
As the current standard of care for treating neovascular AMD,8,9 anti-VEGF agents have resulted in a significant improvement in nAMD prognosis.5,10 The conventional approach to categorizing advanced AMD into treatable nAMD and untreatable GA may soon become outdated, thanks to the emergence of novel GA-targeting therapies. Pegcetacoplan (Syfovre, Apellis Pharmaceuticals) is one such treatment, having shown promising results in reducing GA progression during Phase III trials at the 24-month mark.11
However, it’s worth noting that neovascular AMD and GA can coexist in the same eye, making it particularly important to understand the impact of GA progression on patients receiving anti-VEGF treatment. The worsening of GA can severely affect a patients’ quality of life and long-term visual prognosis.
Moreover, some evidence suggests that anti-VEGF therapy may contribute to the development of macular atrophy and GA.8 The CATT study reported that 20 percent of patients receiving anti-VEGF therapy for nAMD developed GA after two years, doubling to 41 percent after five years.12,13 One possible explanation may be that anti-VEGF treatment can lead to choroidal thinning, which is a known risk factor for macular atrophy.8
But the correlation between anti-VEGF treatment and the development of macular atrophy and GA is still controversial. For this reason, we’ve developed a multistep approach that aims to shed light on this issue. First, we plan to identify the incidence of GA in nAMD patients at different time points using a meta-analysis and meta-regression approach. Second, we aim to report on long-term data regarding GA progression and its correlation with baseline characteristics in patients with nAMD using high-quality multimodal imaging.
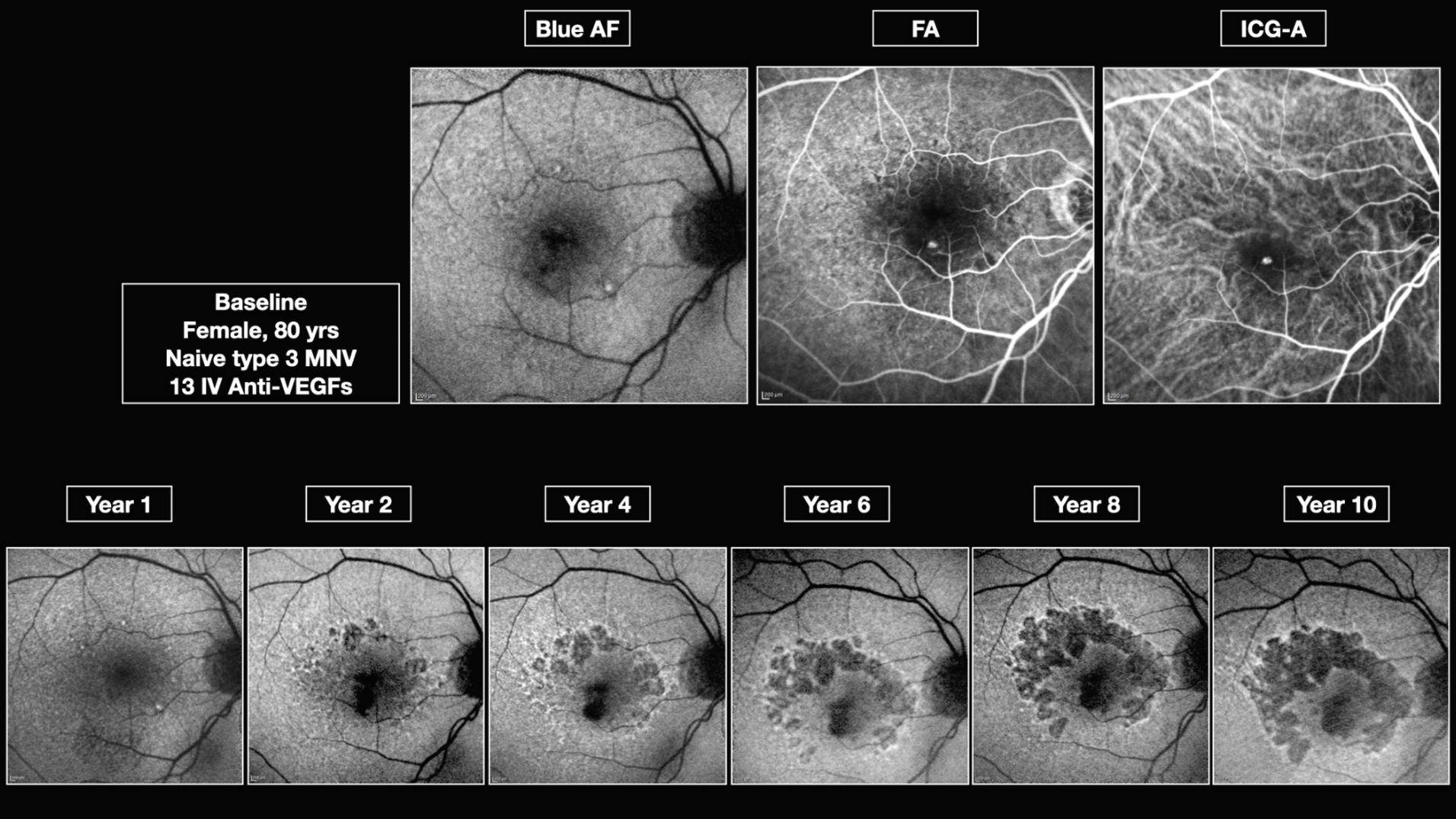 |
| Figure 1: Multimodal imaging demonstrates the geographic atrophy progression over 10 years in an 80-year-old woman with type 3 neovascular membrane. |
Incident GA in nAMD patients: A meta-analysis and meta-regression
The primary objective of our research was to synthesize available evidence on the incidence of new-onset GA during anti-VEGF treatment for nAMD, and to determine whether a possible correlation exists between the use of anti-VEGF agents and the development of GA.
The literature has reported several risk factors associated with AMD and its progression to GA, but no consensus exists about the possible link between the development of GA and treatment with anti-VEGFs. We aimed to identify and investigate the risk factors associated with GA progression, comparing pooled data with the natural history of AMD.
We conducted a meta-analysis at different time points using data from large published studies on incident GA in eyes affected by wet AMD treated with anti-VEGFs, and then compared the incidence rate with that of intermediate AMD that didn’t require anti-VEGF treatment.
Our findings revealed that the incidence of GA in patients with wet AMD treated with anti-VEGFs follows a linear progression, with approximately 17 percent incidence in the first year of treatment, 26 percent in the second year, 34 percent between the third and sixth year and 48 percent in 10 years. The angular coefficient that characterizes the incidence of GA in treated neovascular AMD populations (0.05) and untreated intermediate AMD populations (0.057) is very similar, indicating that there doesn’t seem to be any influence of VEGF inhibition in the development of GA.
Our model also showed that the incidence rate of subfoveal GA was significantly associated with the time interval since the start of treatment, but not with the mean age of patients at the start of anti-VEGF therapy or the number of injections.
However, our study has limitations due to the variability of the selected populations and the differences in the type and regimen of drug administration in each study. In addition, demographic characteristics and ethnicity may have introduced biases in the population considered. Undoubtedly, refined studies using advanced imaging techniques are necessary to comprehensively describe the progression of GA, and to accurately categorize patients based on various subtypes of neovascular membrane.
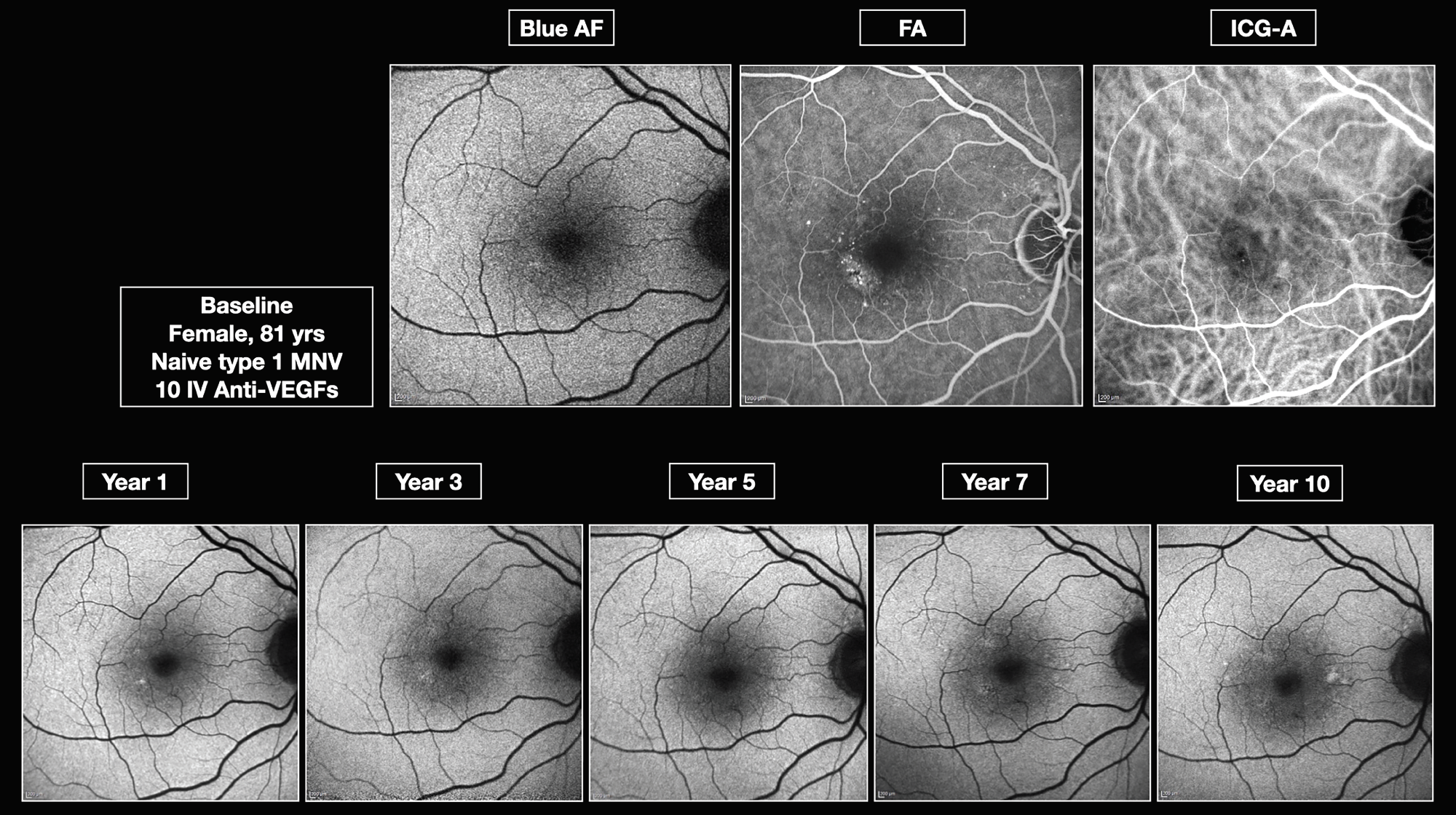 |
| Figure 2. Type 1 choroidal neovascularization without geographic atrophy development over a 10-year period. |
Long-term progression of GA in nAMD: A retrospective analysis with high-quality multimodal imaging
To assess the long-term progression rate of GA in eyes with nAMD treated with
anti-VEGF agents, we designed a retrospective study. We included eyes that were followed up for five to 10 years and had high-quality multimodal imaging available, including infrared reflectance, optical coherence tomography and fundus autofluorescence. The aim of the study was to report the long-term GA progression rate and to identify risk factors associated with GA progression, specifically focusing on differences between neovascular membrane subtypes.
 |
We included a total of 100 eyes, with a mean follow-up of 8.1 (±2) years. Patients received an average of 22.1 (±11.5) intravitreal anti-VEGF treatments. The study population was evenly distributed between the genders, with an average age of 71.9 (±8.3) years. We found that the overall GA progression rate was 2.1 (±1.9) mm2/year, which is consistent with previous literature reporting a range of 0.53 to mm2/year and a median of 1.78 mm2/year.7
However, the GA progression rate differed significantly depending on the neovascular membrane subtype. We found:
- Type 3 had the fastest growth rate, 3.2 ± 0.70 mm2/year (Figure 1).
- Type 1 had the second-fastest growth rate, 2.4 ±1.12 mm2/year (Figures 2, 3).
- Mixed type followed, 2.2 ±0.69 mm2/year.
- Polypoidal choroidal vasculopathy was next, 2 ±0.53 mm2/year.
- Type 2 had the slowest growth rate, 1.9 ± 0.52 mm2/year.
Our analysis found that demographic characteristics didn’t significantly affect GA progression rate, although intrinsic population characteristics, such as genetics and lifestyle habits, have been shown previously to influence the prevalence of AMD.2
Additionally, we found that the frequency of injections and the type of anti-VEGF agent used for nAMD treatment weren’t associated with GA progression, which seems to be primarily related to the type of neovascular membrane underlying the nAMD.
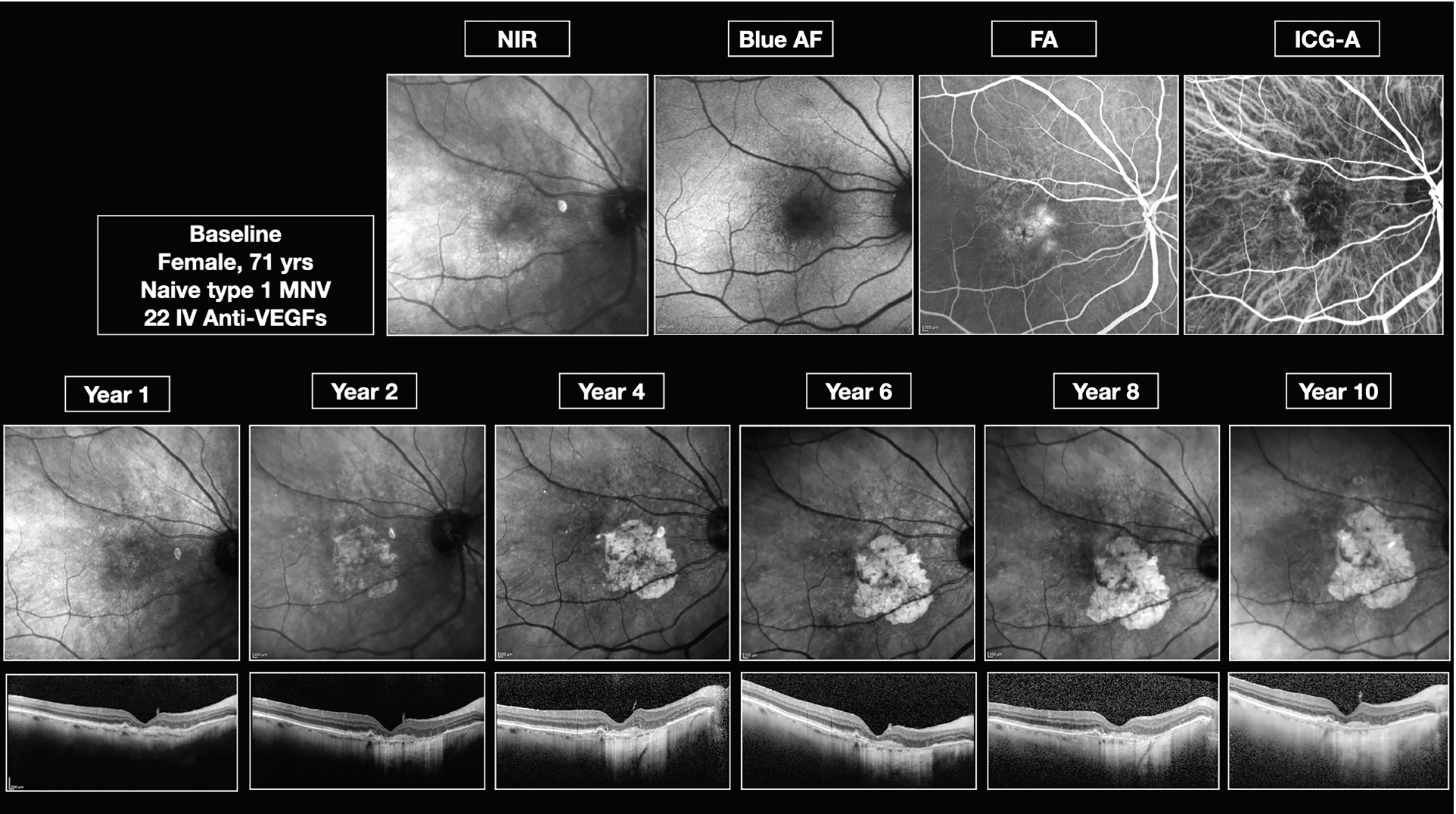 |
| Figure 3. Type 1 choroidal neovascularization with geographic atrophy development over a 10-year period. |
Bottom line
Our findings support the notion that GA is a component of nAMD and that intravitreal injections of anti-VEGF drugs don’t influence the incidence or progression of GA. We observed that the incidence rate of GA in nAMD has a linear increment with an angular coefficient that’s very similar to that observed in populations with intermediate AMD who aren’t treated with anti-VEGFs.
Furthermore, we identified that the incidence and rate of progression of GA in nAMD vary depending on the type of neovascular membrane underlying the maculopathy. Demographic characteristics, which are known to be risk factors for the incidence of AMD in general, didn’t show any correlation with the incidence or rate of progression of GA in our analysis.
GA progression has a devastating impact on the quality of life of patients already undergoing intravitreal treatments for wet AMD, significantly affecting their long-term visual prognosis. It’s crucial that we identify patients at the highest risk of progression, who may benefit from concurrent treatment for both choroidal neovascularization and the loss of RPE and photoreceptor cells in the near future. RS
REFERENCES
1. Schultz NM, Bhardwaj S, Barclay C, Gaspar L, Schwartz J. Global burden of dry age-related macular degeneration: A targeted literature review. Clin Ther. 2021;43:1792-1818.
2. Ricci F, Bandello F, Navarra P, Staurenghi G, Stumpp M, Zarbin M. Neovascular age-related macular degeneration: Therapeutic management and new-upcoming approaches. Int J Mol Sci. 2020;21:1-40.
3. Wong WL, Su X, Li X, et al. Global prevalence of age-related macular degeneration and disease burden projection for 2020 and 2040: A systematic review and meta-analysis. Lancet Glob Health. 2014;2:e106-e116.
4. Daien V, Finger RP, Talks JS, et al. Evolution of treatment paradigms in neovascular age-related macular degeneration: A review of real-world evidence. Br J Ophthalmol. 2021;105:1475-1479.
5. Veritti D, Sarao V, Soppelsa V, Danese C, Chhablani J, Lanzetta P. Managing neovascular age-related macular degeneration in clinical practice: Systematic review, meta-analysis, and meta-regression. J Clin Med. 2022;11:325.
6. Nowak JZ. Age-related macular degeneration (AMD): pathogenesis and therapy. Pharmacol Rep. 2006;58:353-363.
7. Fleckenstein M, Mitchell P, Freund KB, et al. The progression of geographic atrophy secondary to age-related macular degeneration. Ophthalmology. 2018;125:369-390.
8. Foss A, Rotsos T, Empeslidis T, Chong V. Development of macular atrophy in patients with wet age-related macular degeneration receiving anti-VEGF treatment. Ophthalmologica. 2022;245:204-217.
9. Lanzetta P, Mitchell P, Wolf S, Veritti D. Different anti-vascular endothelial growth factor treatments and regimens and their outcomes in neovascular age-related macular degeneration: A literature review. Br J Ophthalmol. 2013;97:1497-1507.
10. Lanzetta P. Anti-VEGF therapies for age-related macular degeneration: A powerful tactical gear or a blunt weapon? The choice is ours. Graefes Arch Clin Exp Ophthalmol. 2021;259:3561-3567.
11. Wykoff CC. Treatment of geographic atrophy secondary to AMD with pegcetacoplan: Two-year outcomes from the randomized Phase 3 DERBY and OAKS trials. Paper presented at American Academy of Ophthalmology Retina Subspecialty Day; Chicago, IL; September 30, 2022.
12. Siedlecki J, Koch C, Schworm B, et al. Enlargement rate of geographic atrophy before and after secondary CNV conversion with associated anti-VEGF treatment. BMC Ophthalmol. 2021;21:4.
13. Grunwald JE, Pistilli M, Daniel E, et al. Comparison of Age-Related Macular Degeneration Treatments Trials Research Group. Incidence and growth of geographic atrophy during 5 years of comparison of age-related macular degeneration treatments trials. Ophthalmology. 2017;124:97-104.



