 |
 |
While hydroxychloroquine retinopathy is generally considered rare, a large study found that the overall prevalence of HCQ retinopathy was 7.5 percent in patients who used HCQ continuously for more than five years, increasing to around 20 percent after 20 years of therapy.1
We describe the main risk factors for HCQ retinopathy, detail the progression of the condition even after drug cessation and summarize the proposed mechanisms of toxicity in this condition.
Reducing risk of HCQ retinopathy
To reduce the prevalence of HCQ, or Plaquenil, retinopathy, the current guidelines for HCQ prescriptions recommend ≤5 mg/kg real body weight. In usage, this translates to a minimum patient weight of 176 pounds to tolerate the typical daily dose of 400 mg. During the first five years of treatment at this recommended level, the risk for HCQ retinopathy development is less than 1 percent.2 At up to 10 years, the rate is less than 2 percent, but it rises to almost 20 percent after 20 years.2
Thus, this more conservative treatment protocol reduces the HCQ toxicity risk early (in the first 10 years), but it doesn’t reduce the risk if the patient has been on therapy for 20 years or more.
Screening for HCQ retinopathy should include multifocal electroretinography and fundus autofluorescence when therapy starts, then yearly after five years of treatment, along with annual spectral-domain optical coherence tomography and 10-2 visual fields after therapy starts (Table).
Here, we report on a case of Plaquenil retinopathy and review the literature.
Case: Progression after halting HCQ
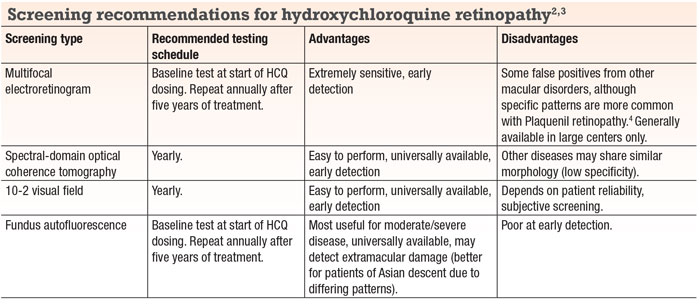
We examined a 56-year-old patient who had used Plaquenil for 13 years and who was noted to have retinopathy. She described having a “sparkly C-shaped” image in her right eye for the previous two years before her first examination.
Despite immediate discontinuation of HCQ, she has continued to have progressively worse night vision and subjective contrast sensitivity over three years of follow-up. The progression is most easily noted in worsening retinal pigment epithelium autofluorescence (Figure 1). Spectral-
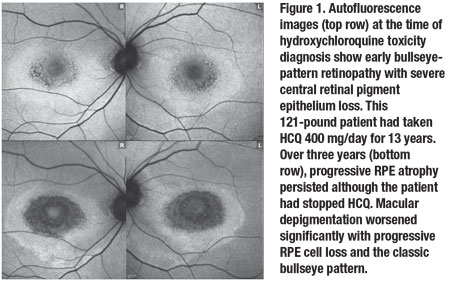
domain optical coherence tomography performed at baseline confirmed generalized retinal thinning with loss of the foveal inner/outer segment junction (Figure 2, page 36).
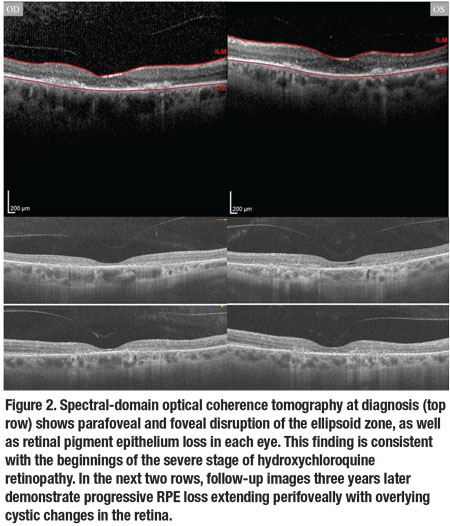
Almost all nine subfields of the macular thickness map for both eyes showed thinning (Figure 3A, page 37). Multifocal ERG confirmed significant loss of foveal function with decreased central waveforms in each eye (Figure 3B).
 |
Qualitative visual changes
We’ve found common symptoms among our HCQ retinopathy patients to be generally limited to the central visual field. They include:
- flashing lights in the center;
- central blurriness;
- central loss of contrast; and
- difficulty reading despite having excellent Snellen acuity.
Mihai Mititelu, MD, MPH, and colleagues studied qualitative vision changes in their HCQ patients.5 Five of their seven patients had complaints relating to night vision and blind spots, and declining visual acuity. Most of the symptoms developed after anatomic changes had occurred.
 |
HCQ mechanisms of action
Hydroxychloroquine is an antimalarial drug often used to help treat rheumatoid arthritis, systemic lupus erythematosus and other autoimmune diseases. It has multiple pharmacological actions, one of which is to interfere with lysosomal function. Lysosomes engage in autophagy, which can lead to the presentation of autoantigens in dysfunctional tissues.
HCQ inhibits lysosomal functions by raising the pH, which inhibits the fusion of lysosomes to autophagosomes.6 This specifically prevents major histocompatibility complex (MHC) class II presentation of autoantigens and CD4+ cell activation. Despite the inactivation of CD4+ cells, HCQ stimulates CD8+ cells by increasing the cross-presentation pathway for exogenous antigens.7 HCQ also inhibits signaling of toll-like receptors (TLRs), preventing antigen-presenting cells (APCs) from presenting antigens and inhibiting them from releasing proinflammatory cytokines such as interleukin-1 (IL-1), IL-6 and tumor necrosis factor (TNF).8
 |
Potential mechanisms of toxicity
HCQ binds strongly to melanin in the RPE and uvea, and is therefore highly concentrated in these tissues—up to 10,000 times the plasma concentration after chronic use.9 A primary mechanism of action for HCQ is that it interferes with lysosomal function, specifically autophagy. It interferes with the ability of RPE cells to digest photoreceptor residue, leading to accumulation in lysosomes. Oxidation of the lysozymal product results in lipofuscin formation; its accumulation causes RPE dysfunction.10
While the lysosomal toxicity pathway is heavily studied, another proposed toxicity pathway involves the visual cycle. Researchers in Australia and China reported that human organic anion transporting polypeptide 1A2 (OATP1A2) is involved in the uptake of all-trans-retinol (at-ROL) in the RPE.11 RPE recycles the at-ROL and converts it back to 11-cis-retinal.
The same researchers also found HCQ to be an inhibitor of at-ROL uptake by OATP1A2.12 Excess at-ROL accumulation is then converted to lipofuscin. Progressive lipofuscin formation leads to continued lysosomal dysfunction and photoreceptor degeneration.13 The long half-life of HCQ (40 to 60 days) and high concentration in the RPE cells can result in progressive retinopathy long after drug cessation.8
 |
Protecting HCQ-damaged RPE cells
Ruihui Zhang, PhD, and colleagues noted that in-vitro HCQ-exposed RPE cells had close to normal levels of healthy RPE cells when they were treated with salbutamol, an adrenergic beta-2-receptor agonist involved in the protein kinase A (PKA) signaling pathway.
To test the involvement of the PKA pathway, they took these same cells (HCQ-exposed RPE cells + salbutamol) and introduced a PKA inhibitor. The viable RPE cells decreased to levels significantly close to that of RPE cells with HCQ toxicity.
This showed that the cyclic adenosine monophosphate (cAMP)-PKA pathway is involved in the protection of RPE cells.14 They also suggested that beta-2 adrenergic agonists, such as bronchodilators, could be studied to protect against or possibly treat HCQ retinopathy. RS
 |
 |
REFERENCES
1. Melles, Ronald B, Marmor MF. The risk of toxic retinopathy in patients on long-term hydroxychloroquine therapy. JAMA Ophthalmol. 2014;12:1453–1460.
2. Marmor MF, Kellner U, Lai TYY, Melles RB, Mieler WF. Recommendations on screening for chloroquine and hydroxychloroquine retinopathy (2016 Revision). Ophthalmology. 2016;123:1386–1394.
3. Cukras C, Huynh N, Vitale S, Wong WT, Ferris FL 3rd, Sieving PA. Subjective and objective screening tests for hydroxychloroquine toxicity. Ophthalmology. 2015;122:356-366.
4. Maturi RK, Yu M, Weleber RG. Multifocal electroretinographic evaluation of long-term hydroxychloroquine users. Arch Ophthalmol. 2004;122:973-981.
5. Mititelu M, Wong BJ, Brenner M, Bryar PJ, Jampol LM, Fawzi AA. Progression of hydroxychloroquine toxic effects after drug therapy cessation: New evidence from multimodal imaging. JAMA Ophthalmol. 2013;131:1187-1197.
6. Jorge A, Ung C, Young LH, Melles RB, Choi HK. Hydroxychloroquine retinopathy — implications of research advances for rheumatology care. Nat Rev Rheumatol. 2018;14:693-703.
7. Accapezzato D, Visco V, Francavilla V, et al. Chloroquine enhances human CD8+ T-cell responses against soluble antigens in vivo. J Exp Med. 2005;202:817-828.
8. Schrezenmeier E, Dörner T. Mechanisms of action of hydroxychloroquine and chloroquine: implications for rheumatology. Nat Rev Rheumatol. 2020;16:155-166.
9. Browning DJ. Pharmacology of Chloroquine and Hydroxychloroquine, Hydroxychloroquine and Chloroquine Retinopathy. Springer New York; New York, NY: 2014. pp. 35–63.
10. Sundelin SP, Terman A. Different effects of chloroquine and hydroxychloroquine on lysosomal function in cultured retinal pigment epithelial cells. APMIS. 2002;110:481-489.
11. Chan T, Zhu L, Madigan MC, et al. Human organic anion transporting polypeptide 1A2 (OATP1A2) mediates cellular uptake of all-trans-retinol in human retinal pigmented epithelial cells. Br J Pharmacol. 2015;172:2343-2353.
12. Xu C, Zhu L, Chan T, et al. Chloroquine and hydroxychloroquine are novel inhibitors of human organic anion transporting polypeptide1A2. J Pharmaceutical Sci. 2016;105:884-890.
13. Sundelin S, Wihlmark U, Nilsson SEG, Brunk UT. Lipofuscin accumulation in cultured retinal pigment epithelial cells reduces their phagocytic capacity. Curr Eye Res. 1998;17:851–857.
14. Zhang R, Hu DN, Rosen R. Beta-adrenergic agonist protects retinal pigment epithelium against hydroxychloroquine toxicity via cAMP-PKA signal pathway. Int J Ophthalmol. 2020;13:552-559.



