Take-home points
|
 |
|
Bios EDITOR’S NOTE: This article was invited and submitted before Dr. Baumal was appointed chief medical officer of Apellis Pharmaceuticals. Dr. Szelog is a medical retina fellow at New England Eye Center at Tufts Medical Center in Boston. Dr. Baumal is a professor of ophthalmology at New England Eye Center. DISCLOSURES: Dr. Szelog has no relevant disclosures. Dr. Baumal, in addition to the aforementioned title as CMO of Apellis, is a consultant to Genentech/Roche, Regeneron Pharmaceuticals, EyePoint Pharmaceuticals, Ora and Alcon. |
Geographic atrophy affects approximately 5 million people globally and the bilateral form of the disease is responsible for approximately 20 percent of legal blindness in the United States.1,2 Progressive and permanent central vision loss is the end result of GA, characterized by irreversible breakdown of the photoreceptor-retinal pigment epithelium-choriocapillaris complex within the macula.2
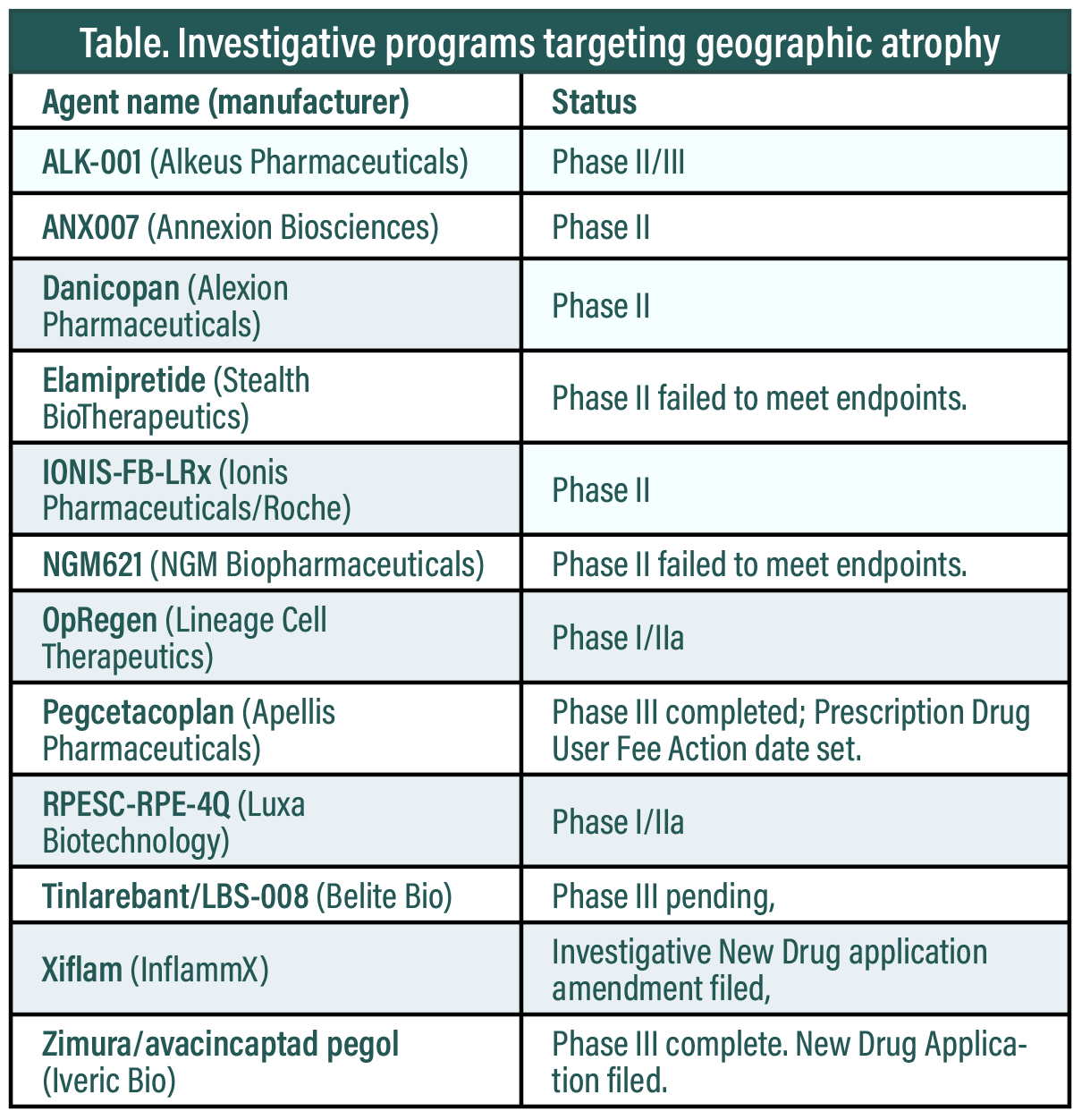
As of this writing, there are no Food and Drug Administration-approved therapies to treat GA, but no less than 12 investigative GA therapies have emerged (Table below). One, pegcetacoplan (Apellis Pharmaceuticals) has the potential to be the first commercially available treatment for GA. The FDA has set an action date of February 26 for its New Drug Application (NDA). An NDA for another, avacincaptad pegol (Zimura, Iveric Bio), was filed in December 2022. (A more comprehensive list of investigative candidates for GA below). This article will focus on the OAKS and DERBY trials of pegcetacoplan that enabled the NDA filing.
Targeting complement pathway
GA often grows around the fovea—i.e., extrafoveal—producing scotomas and affecting reading and driving vision. It ultimately progresses into the fovea with reported growth rates ranging from 0.5 to 2.6 mm2/year, with a median of 1.78 mm2/year.3 The prevalence of GA more than quadruples per decade of life after age 50.4 As a consequence, the overall prevalence of advanced AMD (combined nAMD and GA) has the potential to double over the next 20 years as life expectancy increases.5
The etiology of GA involves genetic factors, which continue to be elucidated, along with environment and age. An optimized diet, healthy lifestyle, smoking avoidance and use of AREDS2 vitamins may confer nominal risk reduction.6 Dysregulation of the complement cascade appears to play a role in GA pathogenesis based on genetic, biochemical and histopathologic evidence. Genetic variants of factors that affect complement component 3 (C3) regulation, such as complement factors B and H, have been associated with advanced AMD.7,8
Multiple therapies targeting the complement cascade have been evaluated. Modes of administration include oral and subcutaneous, subretinal and intravitreal injections. Recent Phase III data for pegcetacoplan and avacincaptad pegol support the hypothesis that altering the complement cascade reduces the rate of GA progression.9,10
 |
|
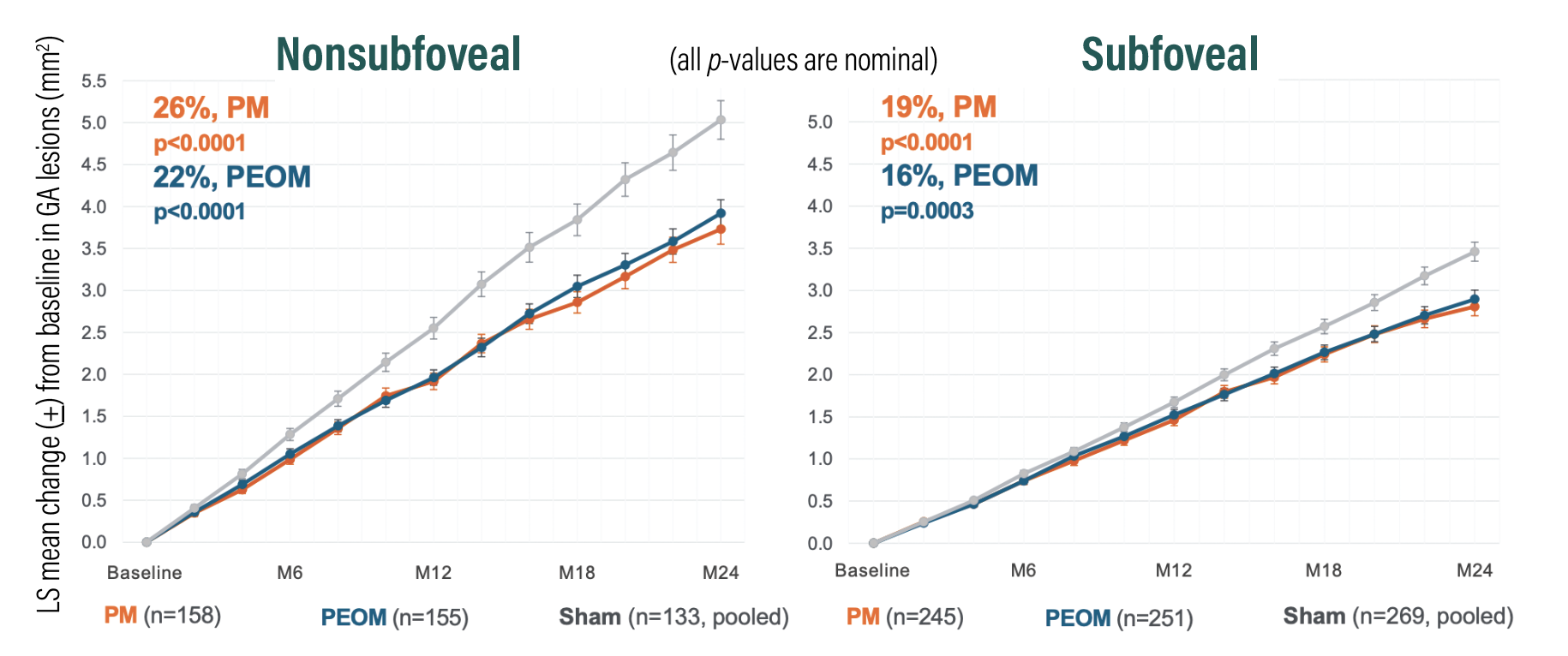
Figure 1. A combined, prespecified analysis from the OAKS and DERBY trials of pegcetacoplan demonstrated 26 and 22 percent reductions in geographic lesion growth with monthly and every-other-month (EOM) dosing, respectively, vs. sham in eyes with nonsubfoveal lesions (p<0.0001 for both); and 19 and 16 percent reductions in lesion growth with the respective dosing intervals vs. sham in eyes with subfoveal lesions (p<0.0001 and p=0.0003). Least square (LS) means were estimated from the mixed-effects model for repeated measures. (Courtesy Apellis Pharmaceuticals) |
Science behind pegcetacoplan
Pegcetacoplan, also known as APL-2, is a pegylated, highly specific, synthetic peptide that binds C3 and C3b, inhibiting downstream effects of inflammation, opsonization and, ultimately, the formation of membrane attack complex (MAC).7 C3 is the central convergence point in the complement cascade of all three activation pathways—classic, lectin and alternative.7
The Phase III DERBY and OAKS trials evaluated the efficacy of pegcetacoplan to inhibit the rate of GA lesion growth and affect visual function. The potential FDA approval of pegcetacoplan would be pivotal in the management of GA. It may provide a tool to reduce GA progression in patients who, until now, have been otherwise defenseless against the threat of inevitable, irreversible vision loss.
 |
Impact on GA lesion growth
OAKS met the primary endpoint of GA lesion size growth reduction at the 12-month mark, with 22 and 16 percent reduction in growth vs. sham in the monthly and EOM treatment groups, respectively (p=0.0003 and p=0.0052).11 In DERBY, both treatment groups narrowly missed statistical significance vs. sham, with 12 percent reduction for monthly and 11 percent for EOM (p=0.0528 and p=0.0750) at the 12-month mark.11
Baseline imbalances in factors that affect GA growth were noted between study arms because DERBY and OAKS enrolled a heterogeneous population of GA eyes with foveal and nonsubfoveal lesions. Combining the DERBY and OAKS study data revealed pegcetacoplan had a strong effect on lesion growth in eyes with extrafoveal lesions for both monthly and EOM dosing vs. sham, with 26 and 23 percent reductions, respectively (p<0.0001 and p=0.0002). This wasn’t unexpected; the natural history of GA demonstrates that nonsubfoveal (extrafoveal) lesions grow faster than subfoveal lesions.
At the 18- and 24-month time points in both studies, pegcetacoplan-treated eyes demonstrated meaningful reduction in GA lesion growth vs. sham. The 24-month data showed increased benefit to pegcetacoplan vs. sham for both monthly (DERBY, 36 percent, p=0.0001; OAKS, 24 percent, p=0.0080) and EOM dosing (DERBY, 29 percent, p=0.0002; OAKS, 25 percent, p=0.0007).12
The design behind DERBY and OAKS DERBY (NCT03525600) and OAKS (NCT03525613) are parallel Phase III, multicenter, double-masked, randomized sham-controlled trials evaluating the efficacy and safety of monthly and every-other-month (EOM) intravitreal pegcetacoplan for reducing progression of geographic atrophy secondary to AMD.23,24 The study excluded patients with GA due to a condition other than AMD, refractive error beyond –6 D of myopia or axial length >26 mm, any history of choroidal neovascularization in the study eye, other active disease-confounding visual functioning, intraocular surgery within three months of randomization or history of macular laser. A history of nAMD in the fellow nonstudy eye was not exclusionary and eyes with subfoveal and nonsubfoveal GA were eligible for inclusion, which differentiates DERBY and OAKS from other GA studies. DERBY and OAKS respectively enrolled 621 and 637 patients with untreated GA, randomized 2:2:1:1 to monthly (n=403) or EOM pegcetacoplan (n=406), or monthly or EOM sham (n= 402, pooled sham group). The primary endpoint for each trial was change in GA lesion area as measured by fundus autofluorescence from baseline to month 12. Additional endpoints included 24-month outcomes of secondary visual function tests, such as change from baseline in monocular reading speed, functional reading independence index score, best-corrected visual acuity, low-luminance deficit, monocular critical print size, National Eye Institute Visual Functioning Questionnaire 25-item Version, distance activity subscale scores, change in GA lesion area and systemic APL-2 concentration over time. Evaluation for ocular/systemic treatment-emergent adverse events was performed through 30 months. Microperimetry was an additional secondary endpoint in OAKS only. |
Diving deeper into findings
The efficacy of pegcetacoplan to reduce GA growth improved over time, most notably between months 18 to 24 (Figure 1). No difference was noted in secondary visual function endpoints between treated and sham groups.
Factors that may have impacted the findings include heterogeneity of the GA population studied, limitations of the current visual function tests (especially in the older, visually compromised study population) and the 24-month duration, which may be too short of an interval to demonstrate a difference.
A post hoc OAKS analysis of microperimetry data evaluating the GA lesion junctional
zone suggested a reduced loss of photoreceptor function in treated eyes.9 Specifically, it found less of a reduction in mean threshold sensitivity within both treatment arms vs. sham at 24 months (0.564 and 0.707 dB higher for monthly and EOM, respectively, p=0.0650, p=0.0202).
Another exploratory post hoc analysis using artificial intelligence to segment optical coherence tomography data showed reduced photoreceptor and RPE loss in pegcetacoplan-treated eyes vs. sham eyes, with a more pronounced and rapid effect on photoreceptor loss.13
Clinical impacts of treating GA
With a therapy effective in slowing GA progression on the horizon, retina specialists will soon have to focus on findings that we previously only observed. We know that BCVA alone is a poor marker of GA progression. Thus, thorough evaluation and monitoring for GA lesions will help to identify patients who will benefit from treatment.
Natural history studies have revealed that GA lesion size progresses and grows around the fovea with retinal destruction, increased scotoma, and functional worsening. However, the center of the fovea that corresponds to BCVA may be preserved until late in the disease. Studies have confirmed that BCVA reflects atrophic changes within the central 1-mm diameter zone of the fovea and correlates poorly with GA lesion size.14,15
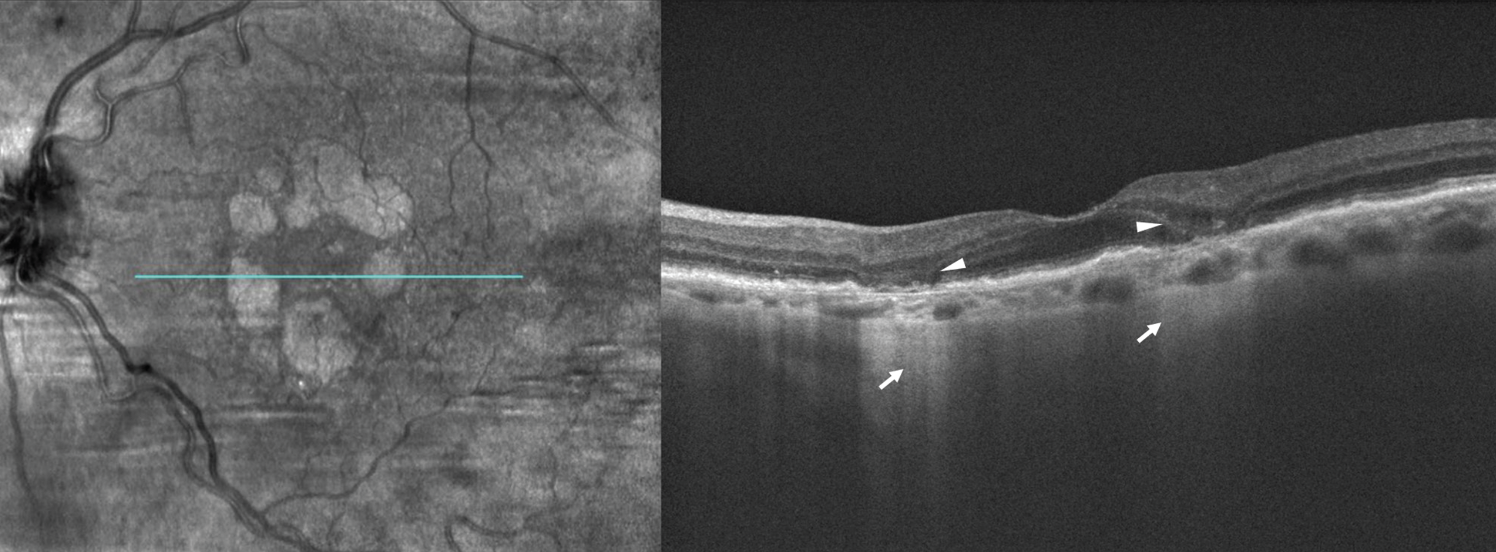 |
| Figure 2. En face optical coherent tomography (left) of the left eye demonstrates multifocal, wreath-like pattern of geographic atrophy wrapping around, but sparing the fovea. Structural spectral domain-OCT (right) reveals hypertransmission of OCT signal through atrophic areas of absent RPE appearing as a bar-code pattern posteriorly (arrows). The diameter of the lesions is greater than 250 µm, meeting the criteria of complete retinal pigment epithelium and outer retinal atrophy (cRORA). Subsidence of inner nuclear and outer plexiform layers and ellipsoid zone disruption overlie areas of RPE atrophy (arrowheads). High-risk features for progression include multifocal GA, foveal-sparing and irregular lesion border. |
Critical role of multimodal imaging
Fundus examination and serial monitoring with multimodal imaging, especially OCT and fundus autofluorescence, will play critical roles in risk-stratification by identifying findings known to be associated with GA progression.16,17
Findings suggestive of elevated GA progression risk include presence of reticular pseudodrusen, junctional zone abnormalities, multifocal lesion configuration and “banded” or “diffuse” lesion presentation.3,18,19 GA in the fellow eye, larger lesion size and extrafoveal lesion location are also predictive of progression.3
Reticular pseudodrusen are subretinal deposits anterior to the RPE and are most often localized to the superotemporal macula. They’re associated with risk of progression from intermediate to advanced AMD, as well as multifocal GA lesion configurations, which tend to progress more quickly than unifocal GA lesions.3 Additionally, regression of reticular pseudodrusen may predict locations of future GA lesions.3
Lesion abnormalities on OCT, FAF
Abnormalities at GA lesion borders as seen on OCT can be classified into three distinct categories:18
Regular, defined as having a smooth margin with no alterations in the outer retina. It’s associated with the slowest progression.
Irregular, which contain severe alterations in the outer retina and irregular margins and are associated with an increased rate of progression.
Splitting, defined as separation of the RPE-Bruch’s membrane complex into inner and outer portions. It may correlate with a progression rate three times faster than lesions with regular borders.
FAF can also help to evaluate GA lesion borders to predict progression based on levels of hyperautofluorescence abutting the lesion.20 They are:
None or minimal change, representing the mildest risk with a median progression of 0.22 to 0.38 mm2/year. These eyes were thus excluded from DERBY and OAKS.19,20
Focal pattern, the presence of a hyperautofluorescent spot <200µm on the lesion border.
Patchy, a spot >200µm.20
Banded, a circumferential ring of hyperautofluorescence surrounding the lesion.
Diffuse, defined as a hyperautofluorescent signal abutting the lesion and extending elsewhere into the adjacent retina.
Median rates of progression with “focal” and “patchy” patterns were 0.56 to 0.81 mm2/year and 1.02 mm2/year, respectively.19,20 “Banded” and “diffuse” patters portend the worst prognosis with progression of around 1.7 to 1.8 mm2/year.19,20
Additional terms to help interpret findings include:
Incomplete RPE and outer retinal atrophy (iRORA), defined on OCT as vertically aligned signs of photoreceptor degeneration, RPE attenuation or disruption, and increased signal transmission into the choroid <250 µm in diameter in the absence of an RPE tear.21,22
Complete RPE and outer retinal atrophy (cRORA), when RPE changes and hypertransmission reach 250 µm the lesion is qualified as cRORA (Figure 2 above).21,22 iRORA lesions are known to progress to cRORA lesions at variable rates from months to years.22
Bottom line
Combined, the DERBY and OAKS data reveal the beneficial effect of intravitreal pegcetacoplan to reduce GA progression vs. sham in eyes with extrafoveal lesions for both monthly and EOM dosing—26 and 23 percent, respectively (p<0.0001 and p=0.0002). Given that extrafoveal lesions are one of several findings suggestive of faster growth rate and more aggressive disease, this is further supportive of this medication’s potential to impact the quality of our patients’ lives. The long-term extension GALE study will provide data on pegcetacoplan use for up to 36 months. RS
References
1. Chakravarthy U, Bailey CC, Johnston RL, et al. Characterizing disease burden and progression of geographic atrophy secondary to age-related macular degeneration. Ophthalmology. 2018;125:842-849.
2. Holz FG, Strauss EC, Schmitz-Valckenberg S, van Lookeren Campagne M. Geographic atrophy. Ophthalmology. 2014;121:1079-1091.
3. Fleckenstein M, Mitchell P, Freund KB, et al. The progression of geographic atrophy secondary to age-related macular degeneration. Ophthalmology. 2018;125:369-390.
4. Rudnicka AR, Jarrar Z, Wormald R, Cook DG, Fletcher A, Owen CG. Age and gender variations in age-related macular degeneration prevalence in populations of European ancestry: A meta-analysis. Ophthalmology. 2012;119:571-580.
5. Wong WL, Su X, Li X, et al. Global prevalence of age-related macular degeneration and disease burden projection for 2020 and 2040: A systematic review and meta-analysis. Lancet Global Health. 2014;2:e106-e116.
6. Lindblad AS, Lloyd PC, Clemons TE, et al. Change in area of geographic atrophy in the Age-Related Eye Disease Study. Arch Ophthalmol. 2009;127:1168-1174.
7. Liao DS, Grossi FV, El Mehdi D, et al. Complement C3 inhibitor pegcetacoplan for geographic atrophy secondary to age-related macular degeneration. Ophthalmology. 2020;127:186-195.
8. Yates JRW, Sepp T, Matharu BK, et al. Complement C3 variant and the risk of age-related macular degeneration. N Engl J Med. 2007;357:553-561.
9. Wykoff CC. Treatment of geographic atrophy secondary to AMD with pegcetacoplan: Two-year outcomes from the randomized Phase 3 DERBY and OAKS Trials. Paper presented at the American Academy of Ophthalmology Retina Subspecialty Day; Chicago, IL; September 30, 2022.
10. Khanani AM, Patel SS, Staurenghi G, et al. GATHER2 Phase 3 efficacy results. Paper presented at American Academy of Ophthalmology Retina Subspecialty Day; Chicago, IL; September 30, 2022.
11. Apellis Pharmaceuticals. DERBY and OAKS Phase 3 top-line results conference call. September 9, 2021. Available at: https://investors.apellis.com/static-files/0abe8e54-7267-47f5-9422-f5d6cda22ae4. Accessed January 21, 2023.
12. Apellis announces 24-month results showing increased effects over time with pegcetacoplan in Phase 3 DERBY and OAKS studies in geographic atrophy (GA) [press release]. Waltham, MA; Apellis Pharmaceuticals; August 24, 2022. Available at: https://investors.apellis.com/news-releases/news-release-details/apellis-announces-24-month-results-showing-increased-effects. Accessed December 4, 2022.
13. Schmidt-Erfurth UM. The role of AI-guided OCT imaging in geographic atrophy. Schmidt-Erfurth UM. Paper presented at the American Academy of Ophthalmology Retina Subspecialty Day; Chicago, IL; October 1, 2022.
14. Heier JS, Pieramici D, Chakravarthy U, et al. Visual function decline resulting from geographic atrophy: Results from the Chroma and Spectri Phase 3 trials. Ophthalmol Retina. 2020;4:673-688.
15. Shen LL, Sun M, Ahluwalia A, et al. Relationship of topographic distribution of geographic atrophy to visual acuity in nonexudative age-related macular degeneration. Ophthalmol Retina. 2021;5:761-774.
16. Boyer DS, Schmidt-Erfurth U, van Lookeren Campagne M, Henry EC, Brittain C. The pathophysiology of geographic atrophy secondary to age-related macular degeneration and the complement pathway as a therapeutic target. Retina. 2017;37:819-835.
17. Sayegh RG, Simader C, Scheschy U, et al. A systematic comparison of spectral-domain optical coherence tomography and fundus autofluorescence in patients with geographic atrophy. Ophthalmology. 2011;118:1844-1851.
18. Lindner M, Kosanetzky S, Pfau M, et al. Local progression kinetics of geographic atrophy in age-related macular degeneration are associated with atrophy border morphology. Invest Ophthalmol Vis Sci. 2018;59:AMD12-AMD18.
19. Holz FG, Bindewald-Wittich A, Fleckenstein M, et al. Progression of geographic atrophy and impact of fundus autofluorescence patterns in age-related macular degeneration. Am J Ophthalmol. 2007;143:463-472.
20. Jeong YJ, Hong IH, Chung JK, Kim KL, Kim HK, Park SP. Predictors for the progression of geographic atrophy in patients with age-related macular degeneration: Fundus autofluorescence study with modified fundus camera. Eye (Lond). 2014;28:209-218.
21. Wu Z, Pfau M, Blodi BA, et al. OCT Signs of early atrophy in age-related macular degeneration: Interreader agreement. Ophthalmol Retina. 2022;6:4-14.
22. Guymer RH, Rosenfeld PJ, Curcio CA, et al. Incomplete retinal pigment epithelial and outer retinal atrophy (iRORA) in age-related macular degeneration: CAM Report 4. Ophthalmology. 2020;127:394-409.
23. ClinicalTrials.gov. Study to compare the efficacy and safety of intravitreal APL-2 therapy with sham injections in patients with geographic atrophy (GA) secondary to age-related macular degeneration.Identifier: NCT03525600. https://clinicaltrials.gov/ct2/show/NCT03525600. Updated June 22, 2022. Accessed January 23, 2023.
24. ClinicalTrials.gov. Study to compare the efficacy and safety of intravitreal APL-2 therapy with sham injections in patients with geographic atrophy (GA) secondary to age-related macular degeneration. Identifier: NCT03525613. https://clinicaltrials.gov/ct2/show/NCT03525613. Updated June 22, 2022. Accessed January 23, 2023.



