Despite the overwhelming success of anti-VEGF agents, challenges remain in the treatment of patients with neovascular age-related macular degeneration, the leading cause of severe vision loss in the elderly population. One common dilemma is the issue of residual intraretinal or subretinal fluid (IRF, SRF) and the uncertainties of treatment with ongoing injections or observation, as these two cases illustrate.
Case 1: Chronic SRF
Mrs. ER was diagnosed with nAMD in her left eye in May 2011. She was 74 years old at the
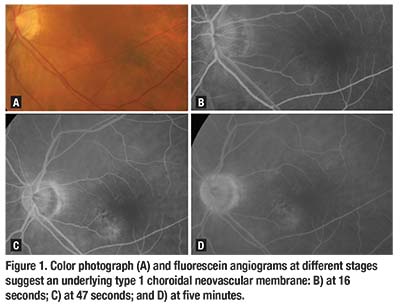 |
time and her initial fundus fluorescein angiogram revealed a type 1 choroidal neovascular membrane (Figure 1). Her presenting best corrected visual acuity was 20/50 in the left eye and she received four initial intravitreal ranibizumab injections (Lucentis, Roche/Genentech), which stabilized her disease.
Following this, we managed her nAMD with an as-occasion-requires (PRN) regimen for the next 30 months. After that, we made the decision to switch to a regular injection regimen because she developed severe visual impairment from end-stage nAMD in the right eye. In total, she received 19 intravitreal ranibizumab injections and 24 intravitreal aflibercept injections (Eylea, Regeneron) in the left eye.escein angiogram revealed a type 1 choroidal neovascular membrane (escein angiogram revealed a type 1 choroidal neovascular membrane (
al fundus fluorescein angiogram revealed a type 1 choroidal neovascular membrane (Figure 1). Her presenting best corrected visual acuity was 20/50 in the left eye and she received four initial intravitreal ranibizumab injections (Lucentis, Roche/Genentech), which stabilized her disease.
Following this, we managed her nAMD with an as-occasion-requires (PRN) regimen for the next 30 months. After that, we made the decision to switch to a regular injection regimen because she developed severe visual impairment from end-stage nAMD in the right eye. In total, she received 19 intravitreal ranibizumab injections and 24 intravitreal aflibercept injections (Eylea, Regeneron) in the left eye.
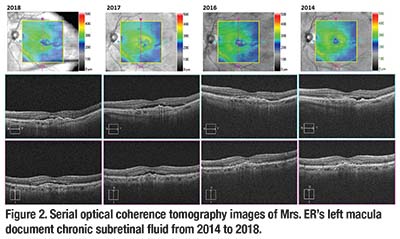
However, the different anti-VEGF injection regimens and the change in the anti-VEGF agent did not make a difference in the appearance of persistent SRF on optical coherence tomography (Figure 2, page 12). Despite enduring chronic SRF for more than six years, Mrs. ER’s left vision was preserved and BCVA was 20/25 in the left eye at her last follow-up visit. She now receives monthly
intravitreal aflibercept injections in her left eye.
Case 2: Persistent, Stable IRF
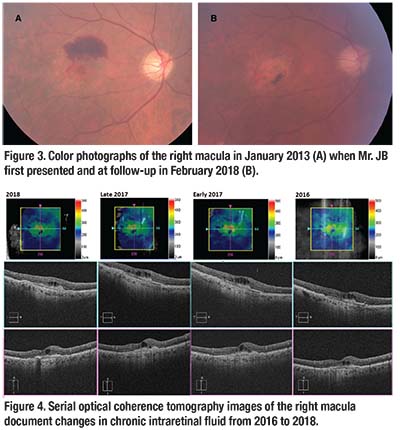
Mr. JB presented with a right macular hemorrhage secondary to nAMD in January 2013 (Figure 3, page 13). He was 82 years old at that time and BCVA was 20/200 in the right eye. Since then, we’ve treated him with regular intravitreal ranibizumab injections every four to six weeks, and he had a total of 44 injections in the right eye. Serial OCT scans showed persistent but stable IRF, associated with underlying subretinal fibrosis (Figure 4, page 13).
Despite regular anti-VEGF injections, he developed atrophic and pigmentary changes in the right macula (Figure 3). BCVA remains at 20/200 in the right eye. He now receives intravitreal ranibizumab injections in the right eye every six weeks.
Why SRF, IRF Drive
Treatment Decisions
The landmark MARINA and ANCHOR studies, which involved monthly anti-VEGF injections, set a high standard for visual acuity results in nAMD treatment.1,2 Subsequent studies have attempted to obtain the same favorable results while treating with a reduced number of intravitreal injections, either with a treat-and-extend or PRN regimen. In almost all of these studies, the presence of fluid on OCT served as a marker of nAMD activity, with both SRF and IRF being associated with disease activity and thus driving decisions regarding the need for retreatment.
Given the heterogeneity of nAMD and its response to treatment, the best management approach varies between patients. A proportion of eyes may never achieve an IRF- or SRF-free macula, even with monthly anti-VEGF injections. However, we now understand that not all areas of hyporeflective signal on OCT represent fluid accumulation from exudative disease. Rather, they may represent chronic degenerative changes or potential anatomical spaces.3 Here, we’ll review studies over the past few years that have shed light on the conundrum of persistent IRF or SRF in patients with nAMD.
Eyes With SRF See Better Than Eyes With IRF
An analysis of the
 |
patients enrolled in the Comparison of Age-related Macular Degeneration Treatments Trial (CATT) found that IRF (cysts) had a much greater negative impact on visual acuity than SRF at all time points analyzed.4 These findings were particularly prominent when the fluid affected the fovea, and were independent of the drug or treatment regimen.
The proportion of eyes with IRF decreased from 76.7 percent at baseline to 48.7 percent at the end of the one-year study across all the treatment groups. The residual cysts tended to be small. The authors hypothesized that the IRF was primarily driven by VEGF-mediated vascular permeability at the start and that non-VEGF-mediated mechanisms, such as apoptotic or necrotic cell death, accounted for the residual IRF at the end of the study.
Supporting this finding in a cohort of 74 nAMD patients with refractory fluid despite monthly ranibizumab injections, researchers at the University of Lausanne in Switzerland reported that IRF was associated with poorer anatomical and functional outcome when compared to SRF.5 Even so, their patients maintained improvement in BCVA, with increases in mean visual acuity of +9, +7.9 and +7.9 letters at months 12, 24 and 36, respectively.
They found that late fluid resolution may also occur. Patients with refractory cysts were more likely to develop fibrosis (odds ratio 3.30), atrophy (OR 3.34) and a 10-letter visual acuity loss (p=0.018).5 Another study of 99 nAMD patients on the treat-and-extend protocol also found that the presence of IRF was associated with poorer vision.6
An additional study utilizing three-dimensional modeling of OCT parameters echoed the finding that IRF is a bad prognostic factor for visual function gain and treatment response in nAMD.7 The amount of IRF at baseline correlated well with baseline visual acuity and the final visual acuity at the end of the one-year study. The study did not, however, find a robust association between SRF, visual acuity and treatment outcomes. The authors concluded that IRF at presentation should be treated aggressively until maximum resolution is achieved, as resolution of IRF resulted in better vision gain.
Why Eyes with SRF See Better
In contrast, the presence of subfoveal SRF may be associated with better visual outcomes. The previously mentioned one-year study of 99 nAMD patients on a treat-and-extend protocol showed the presence of subfoveal SRF had little impact on mean visual acuity.6 Those nAMD patients with persistent SRF also seemed to maintain their vision in the long term.
The Lausanne group retrospectively analyzed 44 nAMD patients with treatment-refractory SRF despite monthly ranibizumab injections over an average follow-up of 32.4 months.8 They found that the mean visual acuity increased by +10.4, +8.2 and +8.6 letters by months 12, 24 and 36, respectively. The refractory SRF was located subfoveally in two thirds of the patients, and 26.7 percent exhibited
complete absorption of SRF after 22.6 months, on average.
The presence of SRF not only seems to positively influence visual function, but also the need for retreatment. The EXCITE trial, which was designed to compare the efficacy of two fixed-treatment regimens (frequent vs. infrequent) of intravitreal ranibizumab in nAMD patients, demonstrated that eyes with SRF at baseline had similarly favorable visual acuity outcomes regardless of the treatment regimen.9
Conversely, eyes without SRF at baseline showed poorer outcomes with infrequent treatment as opposed to frequent injections. Furthermore, improvement in retinal sensitivity during anti-VEGF treatment has been shown to be most pronounced for patients with SRF, whereas patients with IRF benefited to a lesser extent.10
Various investigators have described potential mechanisms for this association between SRF and better visual acuity. Researchers at Manhattan Eye, Ear and Throat Hospital demonstrated that the outer retinal layers in patients presenting with subfoveal SRF are less disrupted than in patients with IRF involving the fovea.11 Furthermore, K. Bailey Freund, MD, and colleagues from the same group considered type 1 neovascularization, with SRF as its predominant form of fluid manifestation, as a more benign subtype of choroidal neovascular membrane in nAMD.12
In a group of nine nAMD patients with type 1 neovascularization and persistent SRF
 |
despite continuous anti-VEGF therapy, the presence of chronic SRF was not associated with visual loss.13 The mean duration of persistent SRF in this group was 5.2 years and long-term visual acuities were 20/40 or better.
Sophie Klimscha, MD, and colleagues analyzed the spatial overlap of IRF, SRF and pigment epithelial detachment (PED) in the OCT scans of 1,341 patients with treatment-naive nAMD using automated segmentation algorithms.14 They found that the locations of PED and IRF are closely related, whereas the locations of SRF did not correlate well to the PED and IRF locations. They hypothesized that in the presence of subfoveal SRF, the neovascular complex, which is represented by the location of PED and IRF, is less likely to involve the fovea. This relationship could explain the beneficial effects of subfoveal SRF and the detrimental effects of fovea-involving IRF.
What Does This Mean In Managing nAMD?
Collating the existing research findings, it appears that we should treat IRF aggressively until it’s stable, and that we can tolerate a degree of persistent SRF. Not all fluid observed in eyes with nAMD reflects active exudation. Persistent intraretinal cystoid changes are known to represent neurosensory degeneration rather than active neovascular leakage, which does not resolve with anti-VEGF injections.
In contrast to IRF, a small residual volume of SRF may be tolerated with minimal negative effect on vision.
Residual SRF may not represent ongoing neovascular activity, but rather a residual anatomical space created by the failure of the neurosensory retina to reattach to the RPE as a consequence of nAMD.
One consequence of insisting upon a complete lack of IRF and SRF is more frequent injections, with the inherent ocular and potential systemic risks associated with them. Another possible ramification is accelerated geographic atrophy. The CATT trial two-year results revealed a 59-percent increase in the risk of geographic atrophy with two years of monthly injections (22.5 injections), regardless of the anti-VEGF drug used, compared with PRN treatment (13.1 injections).15
Furthermore, some evidence shows that SRF could have a protective effect against GA. A sub-group analysis of patients in the CATT trial found that patients with subfoveal SRF greater than 25 µm had a lower risk of GA development.16 Conversely, eyes with IRF involving the foveal center or an area away from it had a higher risk of developing GA than eyes without IRF.
Bottom Line
There remains a need to tailor therapy for individual nAMD patients, and opportunities to further optimize treatment need to be explored and understood. The FLUID study, an ongoing, prospective multicenter trial, is investigating whether patients can tolerate a small amount of SRF with no adverse effect on visual outcome.17 While established OCT biomarkers such as central retinal thickness, presence of subretinal scarring and inner segment/outer segment photoreceptor integrity remain important,18 newer biomarkers such as the presence of SRF and IRF will help retina specialists choose long-term care for their nAMD patients. RS
Dr. Mandelcorn is an assistant professor of ophthalmology at the University of Toronto. Dr. Yap is a vitreoretinal fellow there.
REFERENCES
1. Brown DM, Kaiser PK, Michels M, Soubrane G, Heier JS, Kim RY, et al. Ranibizumab versus verteporfin for neovascular age-related macular degeneration. N Engl J Med. 2006;355:1432-1444.
2. Rosenfeld PJ, Brown DM, Heier JS, et al. Ranibizumab for neovascular age-related macular degeneration. N Engl J Med. 2006;355:1419-1431.
3. Barthelmes D, Sutter FK, Gillies MC. Differential optical densities of intraretinal spaces. Invest Ophthalmol Vis Sci. 2008;49:3529-3534.
4. Jaffe GJ, Martin DF, Toth CA, et al. Macular morphology and visual acuity in the comparison of age-related macular degeneration treatments trials. Ophthalmology. 2013;120:1860-1870.
5. Gianniou C, Dirani A, Jang L, Mantel I. Refractory intraretinal or subretinal fluid in neovascular age-related macular degeneration treated with intravitreal ranizubimab: Functional and structural outcome. Retina. 2015;35:1195-1201.
6. Wickremasinghe SS, Janakan V, Sandhu SS, Amirul-Islam FM, Abedi F, Guymer RH. Implication of recurrent or retained fluid on optical coherence tomography for visual acuity during active treatment of neovascular age-related macular degeneration with a treat and extend protocol. Retina. 2016;36:1331-1339.
7. Waldstein SM, Philip AM, Leitner R, et al. Correlation of 3-dimensionally quantified intraretinal and subretinal fluid with visual acuity in neovascular age-related macular degeneration. JAMA Ophthalmol. 2016;134:182-190.
8. Jang L, Gianniou C, Ambresin A, Mantel I. Refractory subretinal fluid in patients with neovascular age-related macular degeneration treated with intravitreal ranibizumab: Visual acuity outcome. Graefes Arch Clin Exp Ophthalmol. 2015;253:1211-1216.
9. Waldstein SM, Wright J, Warburton J, Margaron P, Simader C, Schmidt-Erfurth U. Predictive value of retinal morphology for visual acuity outcomes of different ranibizumab treatment regimens for neovascular AMD. Ophthalmology. 2016;123:60-69.
10. Sulzbacher F, Roberts P, Munk MR, et al. Relationship of retinal morphology and retinal sensitivity in the treatment of neovascular age-related macular degeneration using aflibercept. Invest Ophthalmol Vis Sci. 2014;56:1158-1167.
11. Sato T, Suzuki M, Ooto S, Spaide RF. Multimodal imaging findings and multimodal vision testing in neovascular age-related macular degeneration. Retina. 2015;35:1292-1302.
12. Freund KB, Zweifel SA, Engelbert M. Do we need a new classification for choroidal neovascularization in age-related macular degeneration? Retina. 2010;30:1333-1349.
13. Bhavsar KV, Freund KB. Retention of good visual acuity in eyes with neovascular age-related macular degeneration and chronic refractory subfoveal subretinal fluid. Saudi J Ophthalmol. 2014;28:129-133.
14. Klimscha S, Waldstein SM, Schlegl T, et al. Spatial correspondence between intraretinal fluid, subretinal fluid, and pigment epithelial detachment in neovascular age-related macular degeneration. Invest Ophthalmol Vis Sci. 2017;58:4039-4048.
15. Comparison of Age-related Macular Degeneration Treatments Trials Research Group, Martin DF, Maguire MG, Fine SL, et al. Ranibizumab and bevacizumab for treatment of neovascular age-related macular degeneration: Two-year results. Ophthalmology. 2012;119:1388-1398.
16. Grunwald JE, Daniel E, Huang J, et al. Risk of geographic atrophy in the comparison of age-related macular degeneration treatments trials. Ophthalmology. 2014;121:150-161.
17. Arnold JJ, Markey CM, Kurstjens NP, Guymer RH. The role of sub-retinal fluid in determining treatment outcomes in patients with neovascular age-related macular degeneration—a Phase IV randomised clinical trial with ranibizumab: The FLUID study. BMC Ophthalmol. 2016;16:31.
18. Schmidt-Erfurth U, Waldstein SM. A paradigm shift in imaging biomarkers in neovascular age-related macular degeneration. Prog Retin Eye Res. 2016;50:1-24.




