 |
Bio Dr. Rajagopal is an associate professor of ophthalmology at Washington University School of Medicine, St. Louis. Dr. Hahn is a vitreoretinal surgeon at NJ Retina in Teaneck, New Jersey. DISCLOSURES: Dr. Rajagopal has no relevant disclosures. Dr. Hahn is a consultant to Alcon and DORC. |
According to the 2022 American Society of Retina Specialists’ Preferences and Trends survey, almost half of U.S. retina specialists—48.8 percent—use indocyanine green as their internal limiting membrane peeling adjuvant of choice, compared to 46.9 percent who use Brilliant Blue and a meager 2.8 percent who use triamcinolone.
I’m among that 2.8 percent, as are several of my colleagues in Saint Louis where I practice. Although I’ve tried ICG and BB many times, I always go back to using Kenalog because I like it better. And I suspect many more than 2.8 percent of you would use it more often if you gave it a fair chance. Here, I explain the advantages of Kenalog and offer some tips on how to use it.
First, the advantages
One agent allows for visualization of the vitreous, epiretinal membranes and the ILM (not to mention residual perfluorooctane bubbles if you use that agent).
It’s cheaper than ICG and BB, especially when using Kenalog as opposed to Triesence (Alcon; yes, there’s a difference) and you should use Kenalog (explanation forthcoming).
The anti-inflammatory effects of residual triamcinolone are often a welcome bonus postoperatively.
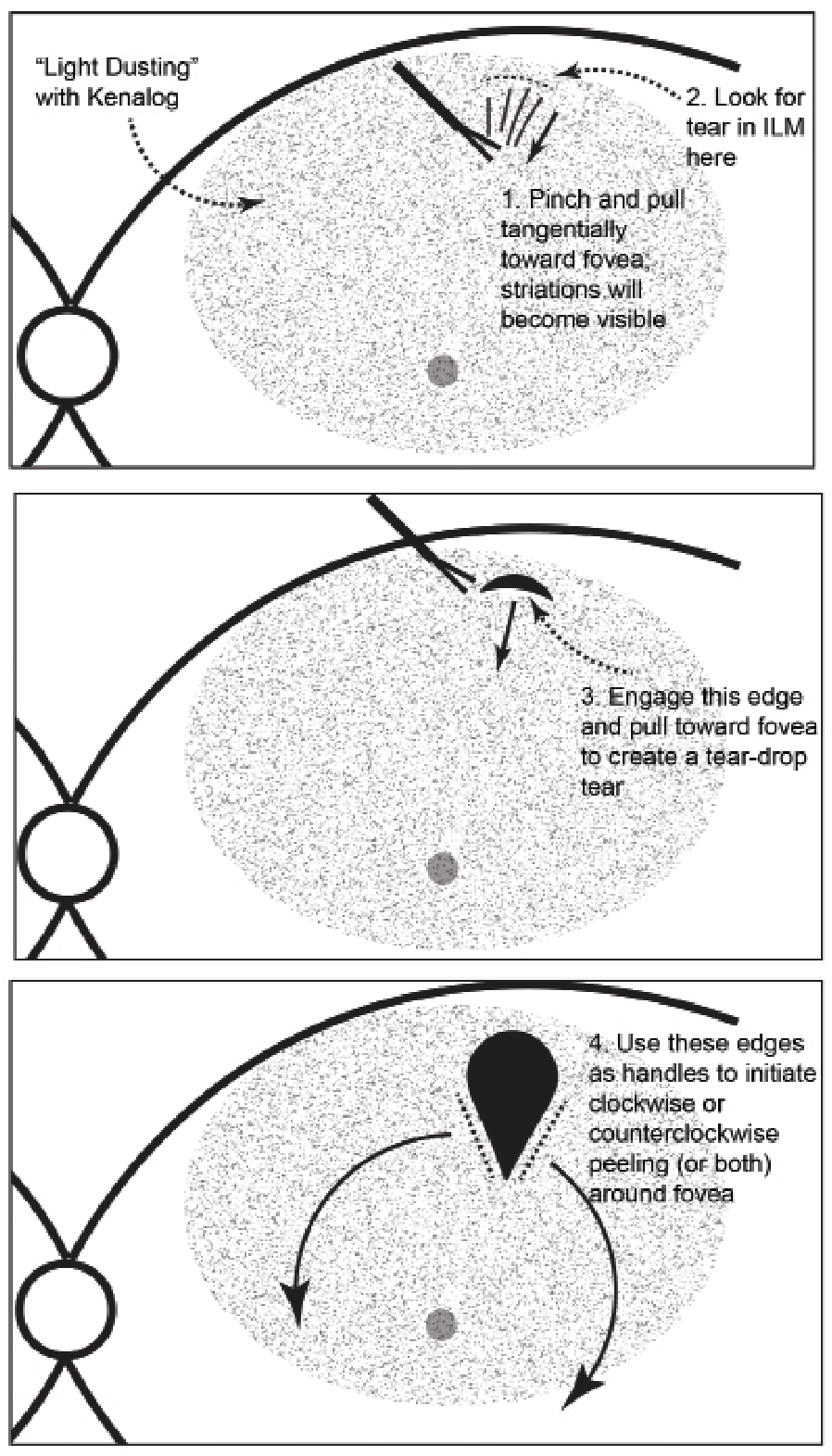 |
| Key steps for peeling internal limiting membrane with Kenalog. Apply a light dusting of Kenalog on the macular surface (1:4 dilution in balanced salt solution). 1. Pinch a distal part of the ILM and pull it toward the fovea tangential to the retinal surface, identifying the tension lines from your instrument. 2. Look for a small rip in the ILM behind the tension line. 3. Release the forceps, grab the newly created torn edge of ILM (this step reduces the risk of pulling at the nerve fiber layer) and pull toward the fovea to create a teardrop-shaped window. 4. Use the lateral edges of the teardrop to serve as handles for extending wide flaps around the entire macular center. Click image to enlarge. |
Kenalog provides superior visualization of the macular “terrain” and helps highlight tangential traction, including traction from your own instruments. This is especially helpful in ILM peeling in combined ERM/ILM situations, proliferative vitreoretinopathy and tractional retinal detachment.
And there’s much less concern for toxicity with Kenalog than ICG.
Tips on how to use Kenalog
Concentration is key. Aim for a “light dusting” of the macular surface. Too little, and you may as well do a naked peel. Too much, and you lose all visualization akin to being in a heavy snowstorm. We prefer a 1:4 dilution of Kenalog (directly from the bottle) mixed with sterile balanced salt solution. Avoid having circulator nurses remove the preservative, as that step is an unnecessary potential source of contamination or needle-stick injury. During the course of vitrectomy, the preservative gets washed away and seldom causes any inflammation.
Use Kenalog, not preservative-free Triesence. It’s not (all) about cost. Kenalog particles are small and perfectly granular. They allow for the ideal amount of dusting. Triesence tends to settle in clumps and when it does so it becomes a poor visualization aid, although it probably works fine for vitreous staining.
ICG-stainers contend that Kenalog can’t identify the ILM. Not true. Just look for the characteristic features: blanching of the peeled macular tissue; petechial hemorrhages; and scrolling of the peeled membrane. While making the initial flap, look for tension lines from the initial forceps grab, and the subsequent tear in the ILM. That will offer the initial handle for the peel (Figure).
Avoid the pitfall of the infusion washing away the Kenalog granules. Redirect the cannula, lower the infusion pressure or fix any leaks in your valved system. The infusion won’t cause an issue if the system is truly closed, so suture leaky sclerotomies and replace leaky cannulas.
After peeling, remove the excess Kenalog using passive extrusion, especially for macular holes (Video). The granules are easily aspirated in this manner. RS




