 |
Optical coherence tomography may be a key diagnostic tool for evaluating vascular damage in severe COVID-19, according to the author of a small study that demonstrated OCT’s potential to identify a biomarker in patients who’ve had severe COVID-19.
The cross-sectional study, published online in Nature Scientific Reports,1 evaluated 61 eyes in 31 people who had no other comorbidities such as diabetes or heart disease—20 of whom had COVID-19 and 11 healthy controls used for comparison, senior study author William R. Freeman, MD, distinguished professor and director of the Jacobs Retina Center at the University of San Diego in La Jolla, Cailf., tells Retina Specialist Magazine.
The major implication of the study is more about understanding the etiology of COVID-19 and why some patients remain sick with it and less about how it impacts vision, Dr. Freeman says. OCT “may be a way to evaluate the damage that the virus has done to the blood vessels,” he says.
“One of the mysteries of COVID-19 is, how does the virus damage all the organs in the body?” Dr. Freeman says. “People talk about long COVID, which is now becoming epidemic; people get COVID-19, but they don’t fully recover and a lot of them are very disabled. How does it happen?”
 |
|
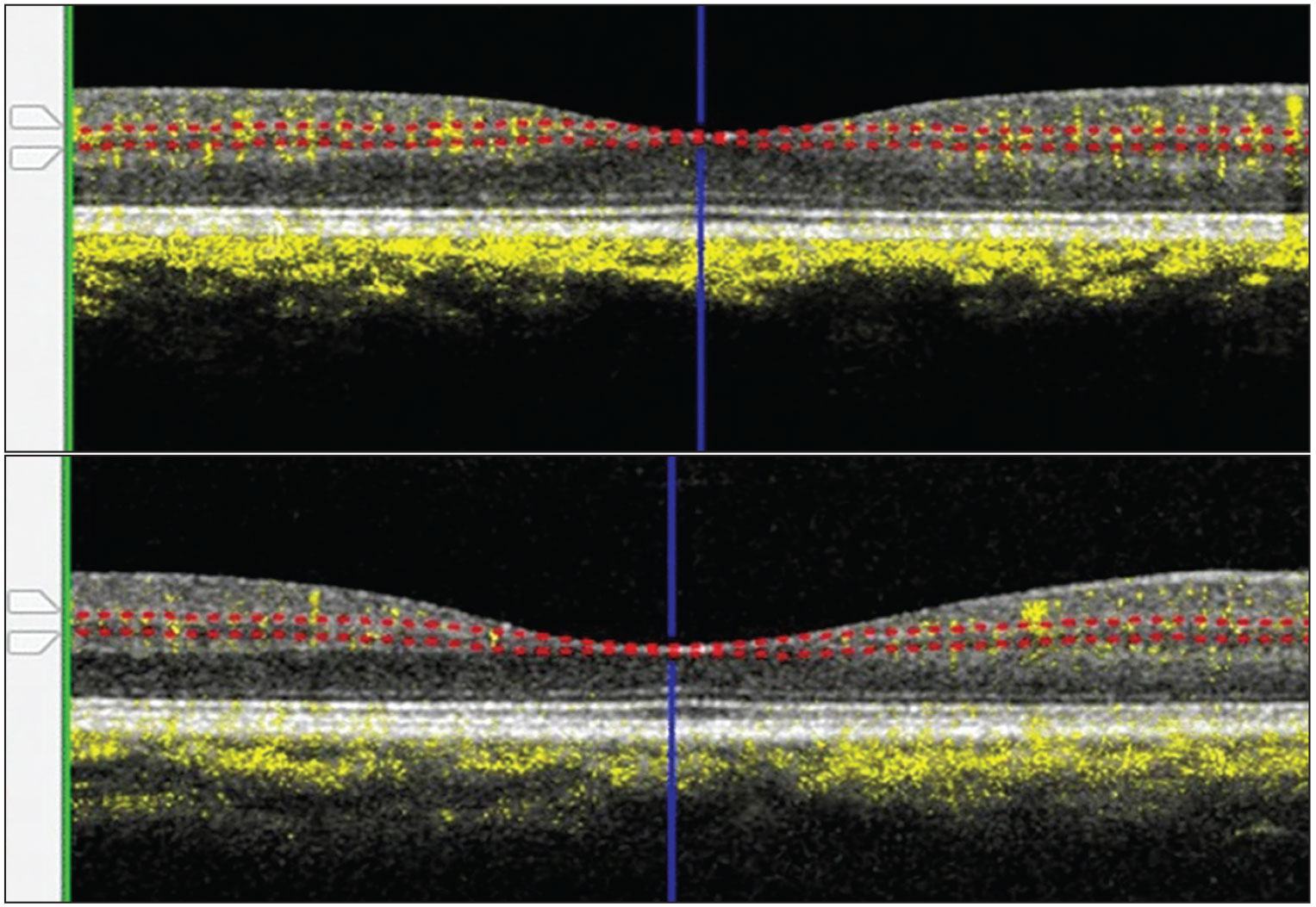
Representative optical coherence tomography images of the intermediate capillary plexus slab of a patient without COVID-19 (top) and one with severe COVID-19 demonstrate corresponding vessel densities of 33 and 18 percent, respectively. (Open access from Kalaw FGP, Warter A, Cavichini M, et al. Retinal tissue and microvasculature loss in COVID-19 infection. Sci Rep. 2023;13:5100. Published online March 29, 2023.) |
Study results
The study found that severe COVID-19 patients had significantly lower mean vessel density from the three inner retinal layers evaluated (24.20, least square mean) than mild COVID-19 patients (26.18, p=0.0057) and controls (26.28, p=0.004). Vessel density in mild COVID-19 patients didn’t differ significantly from normal participants (p=0.87). The analysis used pooled data from the three measured inner retinal layers.
The severe COVID-19 patients demonstrated the following findings from the three specific measured retinal layers:
- Significantly decreased retinal volume in the outer 3 mm of the macula (p=0.02).
- Significantly lower total retinal vessel density (p=0.0004 and 0.0057 in controls and the mild COVID-19 group, respectively).
- Significantly lower intermediate (Figure) and deep capillary plexuses (p<0.05).
“So it seems that it’s not only a viral pneumonia that typically resolves, but the virus damages the blood vessels in many organs in the body,” Dr. Freeman says. “In ophthalmology—in retina—we’re able to visualize microscopic vessels, living vessels, measure the blood flow into those vessels in living patients to an accuracy that’s not possible anyplace else in the body.”
That’s because of OCT. The study patients had a comprehensive opthalmologic examination that included retinal imaging obtained from spectral-domain OCT and evaluation of retinal vessel density with OCT angiography.
OCT may solve the mystery
This may mean that ophthalmologists are uniquely positioned to evaluate COVID-19-related vascular changes, Dr. Freeman says.
He notes that pulmonologists have reported that COVID-19 causes vascular damage to the lungs and that multiple reports have been published of COVID-19 patients who’ve had reduced cognition. “That is felt to be potentially due to damage in the vessels in the brain,” Dr. Freeman says.
However, there’s no readily available way to image those vessels, he adds. “OCT may be a way to evaluate the damage that the virus has done to the blood vessels,” he says. “The eye, and the retina, is a window into the brain and into the microcirculation everywhere.”
What’s unique about this study was that it enrolled only COVID-19 patients that had no previous comorbidities, Dr. Freeman says. “This is the first observation, I would stress: that with all the problems with the other studies, we eliminated the confounding problems,” he says. “We believe the loss of the vessels in the retinal tissues is real. It’s objectively seen on scans.”
This doesn’t mean that retinal changes brought on by COVID-19 impact visual acuity. “This is not causing visual symptoms because they’re relatively small changes and there’s a lot of redundancy in the retina. You can lose a few cells and you’ll still see,” he says.
“You’re not going to go blind from this, but it is an objective marker. It’s a scan that can be done on a nondilated patient and this may tell us who’s likely to get long COVID and who will have COVID complications,” Dr. Freeman says.
Future research should aim to repeat the study, he says. “This was a difficult study to do because you have to eliminate people with other underlying conditions.”
Dr. Freeman has no relevant relationships to disclose.
— Richard Mark Kirkner
REFERENCE
1. Kalaw FGP, Warter A, Cavichini M, et al. Retinal tissue and microvasculature loss in COVID-19 infection. Sci Rep. 2023;13:5100. Published online March 29, 2023. Available at: https://www.nature.com/articles/s41598-023-31835-x#citeas. Accessed May 10, 2023.
In Brief The Food and Drug Administration approved Mydcombi (tropicamide/phenylephrine, Endogena Therapeutics reports completing patient enrollment ahead of schedule in its Phase I/IIa trial of EA-2353 for retinitis pigmentosa. The trial has enrolled 14 patients. The FDA accepted the supplemental Biologics License Application for additional indication for Vabysmo (faricimab-svoa, Genentech/Roche): macular edema following retinal vein occlusion. The FDA approved an Investigational New Drug application submitted by Atsena Therapeutics for a Phase I/II clinical trial of the gene therapy ATSN-201 for X-linked retinoschisis. The FDA granted Breakthrough Device Designation for the Prima System (Pixium Vision), a subretinal, miniature photovoltaic wireless implant, for dry age-related macular degeneration. |



