 |
In degenerative retinal diseases, particular cell types die. These include retinal ganglion cells (RGCs), retinal photoreceptors (PRs) and retinal pigment epithelium (RPE) cells, and they do not appreciably regenerate to restore lost function. Stem cells are an attractive source of cell therapy. They harbor the key ability to self-renew (i.e., make more copies of themselves) and differentiate (i.e., form into specialized cell types like RPE).1 In this way stem cells can generate clinically relevant amounts of the cell types lost in disease.
Progenitor cells are similar to stem cells; however, their ability to self-renew or differentiate into multiple cell types is more limited. Stem cell therapy is classically considered to be one type of cell therapy, in which clinicians use stem/progenitor cells to produce differentiated cells such as RGCs, PRs and RPE in vitro or in vivo. However, many stem cells also produce a multitude of proteins (also called cytokines), some of which promote the survival of dying RPE and PR cells that are dysfunctional but still alive in AMD. This could theoretically slow the progression of retinal degeneration, even if the stem cells do not replace the dying cells or restore function.2
Here, we review the types of stem cells used for treatment of retinal diseases and the findings of some past studies, and we explore the early results and safety concerns related to this technology.
Types of Stem Cells
Cell therapy uses three classes of stem/progenitor cells: pluripotent stem cells (PSCs); fetal cells; and postnatal/adult cells (Figure 1, page 40).
Many cell-based therapies for retinal diseases use PSCs, which can form in any tissue of the body. Because they can self-renew indefinitely, PSCs could generate nearly unlimited amounts of differentiated retinal tissues.1 The most common stem cells currently employed for the treatment of retinal diseases are two types of PSCs—human embryonic stem cells (hESCs) and induced pluripotent stem cells (iPSCs)3—and a non-pluripotent cell type, so-called “adult” stem cells.
 |
Embryonic stem cells are pluripotent and are cultivated from the inner cell mass of a five-day-old blastocyst,1 while iPSCs are PSCs derived from reprogrammed differentiated somatic cells, such as adult skin fibroblasts (connective tissue near the skin) or white blood cells.3 These hESCs and iPSCs can then be converted to neural retinal or RPE cells.
Other classes of stem/progenitor cells that have been used in trials for retinal diseases are derived from the fetal central nervous system—the developing brain, spinal cord and retina.4 Fetal retinal stem/progenitor cells build the retina during embryonic development through limited self-renewal and tissue-specific differentiation.5
So-called “adult stem cells” are post-natal cells that can generate some, or all, of the cell types of the organs from which they originate. For instance, hematopoietic stem cells are derived from bone marrow and can reconstitute all the cells of the blood (red and white cells, platelets, etc.), and are used in patients with blood cancers or immunodeficiencies.6 In fact, hematopoietic cell transplantation is currently the only Food and Drug Administration-approved cell therapy.6,7
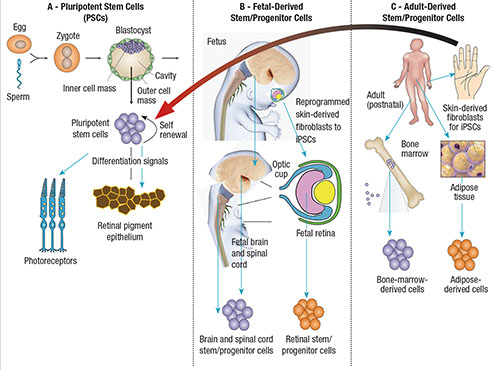 |
| Figure 1. Cell therapy uses three classes of stem/progenitor cells: pluripotent stem cells (PSCs); fetal cells; and postnatal/adult cells. PSCs (A) are derived from the inner cell mass of blastocysts (five-day-old embryos) or from reprogrammed skin cells (arrow from C). They can be differentiated to photoreceptors (not yet used in clinical trials) and retinal pigment epithelium (currently in use in clinical trials). Fetal stem/progenitor cells (B) are derived from the fetal central nervous system, such as the developing brain, spinal cord and retina.4 A few clinical trials have used fetal retinal stem/progenitor cells, which “build” the retina during embryonic development.5 In many cases, these cells do not actually replace dying retinal cells, but could indirectly support survival of host retinal cells through secretion of pro-survival proteins known as cytokines. Adult stem cells (C) can generate some, or all, of the cell types of the organs from which they are harvested. Various clinical trials have proposed use of bone-marrow-derived cells for a variety of retinal disorders.8 In these cases, these cells do not actually replace dying retinal cells, but could indirectly support survival of host retinal cells through secretion of pro-survival cytokines. |
Bone Marrow Cell Therapy
The “Holy Grail” of stem cell therapies is the replacement of dead or dying retinal cells with stem-cell-derived cells to restore vision. Indeed, in bone marrow transplantation, cells produced from donor bone marrow partially replace the recipients’ blood system, leading to restoration of the immune system and other functions fundamental to the hematopoietic system, like oxygenation.
However, in the human central nervous system, which includes RGCs, there is little evidence that stem cells or stem-cell-derived cells can themselves produce the missing or dying cell types following transplantation. One exception is PSC-derived RPE; early phase clinical trials have shown patches of increasing pigmentation after transplantation of donor-derived RPE into the subretinal space.9 Still, to date there is no evidence that stem-cell-derived cells, such as RPE, can improve or restore vision. Further study and larger, prospective trials will be needed.
If stem cells or stem-cell-derived cells cannot produce the missing cell types and integrate into the host retina to restore function, why consider stem cells as a route to therapy? Stem cells, like other cells, are cytokine-producing factories. These cells secrete growth factors that may improve the survival and function of host cells.2 Thus, salutary effects of cell therapies, such as stem-cell transplantation in the human retina, may be secondary to this indirect effect, rather than direct replacement of dying retinal cells with those derived from stem cells.
There are three methods for delivering stem cells (Figure 2, page 42).
To date, only results of early phase stem/progenitor cell therapy trials have been reported. In general, most trials were Phase I or IIA, and not powered to detect efficacy. The primary goal of these trials has been to determine whether these interventions are safe. It is important to note that none of these trials included control groups, although they did monitor untreated fellow eyes.
PSC-based Trials
Several PSC-based trials are in progress. Ocata Therapeutics, acquired earlier this year by Astellas Pharma, was among the first to conduct PSC-based trials in humans. These include Phase I/II trials of human ESC-derived RPE for dry AMD, Stargardt disease and myopic macular degeneration in the United States, United Kingdom and Korea. A recent report detailing two trials that used systemic immunosuppression (tacrolimus and mycophenolate mofetil) in combination with subretinal transplantation in human ESC-derived RPE for dry AMD (nine patients) and Stargardt disease (nine patients), showed increased subretinal pigmentation at the border of atrophic lesions, consistent with subretinal RPE transplantation, in 13 of 18 patients.9 The median follow-up was 22 months.
In the 18 studied eyes, best-corrected visual acuity improved in 10 eyes, remained the same in seven eyes and decreased by more than 10 letters in one eye. Untreated fellow eyes did not have similar improvements in visual acuity. Consistent with findings from pre-clinical studies, these authors reported no correlation between increased subretinal pigmentation and improvement in vision.9 Importantly, while these trials suggest a biologic effect, larger studies are needed to detect a true effect.
Local adverse events included cataract progression, and separate, single cases of focal RPE loss at the injection site, epiretinal membrane and vitreous inflammation with intravitreal membrane formation and Staphylococcus epidermidis endophthalmitis that resolved two months after intravitreal antibiotic therapy.9
Other serious adverse events included hemiparesis, chest pain, femoral neck fracture, mental status change and skin cancers, some of which may have been unrelated to the treatment or due to systemic immunosuppression.9 Importantly, these trials did not detect any tumor formation. A Korean study recently reported similar results in four patients (two with dry AMD and two with Stargardt disease).10
The first ever iPSC-based intervention to be tested in humans is a recent trial for wet AMD, at the RIKEN Institute in Japan. In September 2014, researchers injected autologous iPSC-derived RPE subretinally into a woman with wet AMD.11 The patient had previous anti-VEGF injections, and the procedure the authors described involved resection of subretinal fibrotic tissue prior to subretinal injection of the cells. The autologous iPSCs were originally derived from the patient’s own skin fibroblasts. Short-term safety data suggested that the procedure was safe.
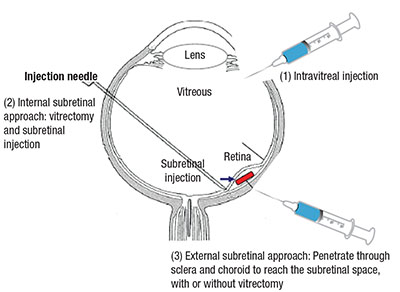 |
| Figure 2. Investigators have employed three methods of intraocular delivery of cell therapies: intravitreal; internal subretinal; and external subretinal. The internal subretinal approach accesses the subretinal space intraocularly (usually after vitrectomy) while the external subretinal approach accesses the subretinal space via the choroid and sclera. |
However, on reprogramming skin fibroblasts to iPSCs in the second patient, the authors detected genomic alterations (mutations and copy number variations) not present in the original cells. Theoretically, such mutations could increase the risk of tumor growth from the iPSC-RPE cell. In response, the RIKEN Institute halted the autologous iPSC-RPE trial in 2015.12 As of June 2016, the investigators planned to resume the trial, but they will no longer use autologous cells derived from the patient’s own skin, reprogrammed to iPSCs, differentiated to RPE and then transplanted into the same patient. Instead, banked, allogeneic iPSCs will replace the autologous iPSCs as the source for RPE.13
Other studies, such as a trial sponsored by Pfizer, will attempt to grow PSC-derived RPE on a scaffold and then transplant the RPE-scaffold subretinally.
Fetal Stem/Progenitor Cells
While no studies of fetal-derived stem/progenitor cell therapies have been published, abstracts have described the subretinal transplantation of human fetal spinal cord and brain-derived central nervous stem cells (HuCNS-SCs) in geographic atrophy in a 15-patient, open-label Phase I/II study.4 A prospective analysis showed an increase in subfield thickness and macular volume in the treated eye vs. the untreated eye, as well as slowed growth of geographic atrophy. However, a reading center’s post-hoc analysis did not confirm the latter findings. Few details on adverse events are available, and with the dissolution of the company sponsor, Stem Cells Inc., it remains to be seen whether development of this technology will continue.
Importantly, the investigators have made no claim that the HuCNS-SCs actually differentiate to RPE or photoreceptors, but instead may slow GA indirectly through secretion of cytokines that promote survival of the recipient’s RPE.
Massachusetts Eye and Ear and Harvard Medical School recently initiated a fetal retinal progenitor cell transplant trial. Unlike the PSC-based trials, and similar to the brain/spinal cord fetal neural stem cell trials, the cells are not differentiated to mature cells such as RPE or photoreceptors; rather, they are injected as precursor cells.15 This work involves subretinal transplantation of fetal retinal progenitor cells in a study sponsored by ReNeuron Group as part of a Phase I/II trial for advanced retinitis pigmentosa. Clinical and safety data are not yet available. The company and study investigators hope to see that the fetal retinal progenitor cells improve vision by directly differentiating to photoreceptors or by an indirect effect: secretion of factors that promote survival of host retinal cells.
Another company, jCyte, is slated to begin its first fetal retinal progenitor cell transplants through intravitreal injections. The rationale is that the fetal retinal progenitor cells will clump in the vitreous and secrete factors that will slow retinitis pigmentosa rather than migrate to the retina and differentiate to mature retinal cells.16
It is important to note that the rationale in all of these fetal stem/progenitor retinal transplant trials is not necessarily to replace dying RPE and photoreceptors with stem cell-derived RPE and photoreceptors. Instead, any actual biological effect would likely be an indirect one, perhaps through the secretion of cytokines to promote survival of the recipients’ own retinal cells. This approach stands in contrast to PSC-based RPE trials in which the goal has been to actually replace the dying or dead RPE with PSC-derived RPE and restore vision.
‘Adult’ Stem/Progenitor Cells
By far the most common “stem cell” trials for retinal diseases are sourced from often heterogeneous cell populations known as “adult” stem/progenitor cells—postnatal cells isolated from the individual sometime after birth.
For instance, the umbilical-tissue-derived cells used in Centocor Inc.’s trial are isolated from umbilical tissue present immediately after birth. Other cell types such as adipose/fat tissue are typically isolated from an adult patient. Umbilical tissue, fat, white blood cell2s and many other cell types used in these trials are ultimately bone-marrow-derived.
The rationale of using bone-marrow-derived cell transplants for retinal disease is not well understood, but preclinical models have suggested that these cells secrete cytokines that might preserve retinal cells through actions on the cells themselves and/or by stabilizing retinal vessels.17 It is important to keep in mind that the traditional animal model for proliferative vitreoretinopathy (PVR) is to inject bone-marrow-derived cells (plasma) into the vitreous.18 Therefore, some of the adverse events reported in some of the trials we review here appear to be consistent with the interventions known to produce to PVR in animal models.
These bone-marrow-derived adult stem/progenitor cell types do not generate retinal tissues. Therefore, unlike PSC-based trials but similar to fetal stem/progenitor-cell-based trials, the potential biological effect from adult stem/progenitor trials would be due to cytokine release that promotes retinal cell survival, rather than directly replacing dying or dead RPE or photoreceptors.
Bone-Marrow-Derived Cells
More than 10 bone-marrow-derived cell therapy trials for retinal diseases are currently listed at clinicaltrials.gov, but here we will review only some of these trials based on the availability of published reports.
Investigators at the University of California, Davis, reported on six-month data of six eyes in a trial involving intravitreal injection of the CD34+ fraction of autologous bone- marrow-derived cells for retinal vascular occlusion, dry AMD or retinitis pigmentosa.19 There was no visual benefit, no improvement and no worsening of the electroretinogram (full-field and multifocal), and the cells were linked to hyper-reflective macular deposits on adaptive optics optical coherence tomography in one patient. The authors reported no adverse local or systemic side effects.19
Another trial, by MD Stem Cells, involved intravitreal, retrobulbar, sub-Tenon’s, subretinal and intra-optic nerve injections of autologous bone-marrow-aspirate-derived cells for “glaucoma, ischemic optic neuropathy, optic atrophy, optic neuritis and some trauma.” The so-called Stem Cells Ophthalmology Treatment Study (SCOTS) is self-described as “the largest ophthalmology stem-cell study registered at the National Institutes of Health to date.” In a June 2015 case report, SCOTS investigators reported marked bilateral vision improvements in one woman with idiopathic optic neuritis who received intravitreal injections of bone-marrow-derived cells.20 The authors reported no adverse events in this patient apart from “tearing and conjunctival ecchymosis.”
A few months later, SCOTS investigators reported marked bilateral improvements in vision in a woman with relapsing optic neuritis who received vitrectomy with injection of autologous bone-marrow-derived cells into the optic nerve of the right eye, and retrobulbar, sub-Tenon’s and intravitreal injections of the same in the left eye.21 A June 2016 case report by non-SCOTS investigators described their findings and intervention in a man with a history of Stargardt disease who developed proliferative vitreoretinopathy with a recurrent retinal detachment following treatment in the SCOTS trial.22
The patient originally underwent a pars plana vitrectomy and subretinal injection of autologous bone marrow-derived cells in the right eye at another facility. A month later, he underwent an intravitreal injection of similar cells in the left eye. He developed a retinal detachment and was treated with a scleral buckle, cryopexy and external drainage of subretinal fluid at the SCOTS facility. He was referred to another facility for recurrent retinal detachment due to proliferative vitreoretinopathy (PVR) and underwent pars plana vitrectomy, pars plana lensectomy, membrane peel, endolaser, fluid-air exchange and silicone oil injection, at which time the retina was reattached and the vision improved to 20/300.22
Another case report involved a woman with retinitis pigmentosa who developed PVR/thick epiretinal membrane (ERM) following intravitreal injection of “autologous stem cells.” Following vitrectomy and partial peeling of the ERM, histopathological analysis revealed the presence of CD34+ cells, likely from bone marrow-derived cells.23
Umbilical-tissue-derived cells, which contain a mix of mesenchymal stem cells, placenta-derived calls and dermal fibroblasts, are isolated from the neonatal umbilical cord. Janssen Biotech is conducting a trial using a microcatheter through the sclera and choroid to deliver umbilical-tissue-derived cells to the subretinal space for geographic atrophy.24 Since these umbilical cells do not generate retinal tissue, the theoretical mode of effect would be indirect, through secretion of cytokines that might preserve the recipient’s retinal cells. As yet, there has been no formally published report on this trial.
Autologous adipose-derived cells, collected from liposuction, have been proposed as intravitreal cell therapy for dry AMD. Bioheart Inc. sponsored a study using this approach, but the study has since been suspended. A similar trial in Russia is currently enrolling patients with open-angle glaucoma; it will involve sub-Tenon’s administration of adipose-derived cells. To our knowledge, no reports have been published on the results of this or other adipose-cell-based trials.
While no FDA-approved stem cell treatments for retinal diseases are yet available, the evidence from early phase trials supports feasibility. There remain important caveats, however, including uncommon but serious adverse events such as endophthalmitis, PVR and retinal detachment.
To date, no Level One evidence exists to support that these therapies improve vision, but it is important to keep in mind that these early phase trials are not powered to detect efficacy. Larger, prospective and controlled trials are needed to determine whether statistically significant, meaningful visual improvements are possible with cell therapy strategies. The answers to these crucial questions should arrive soon enough. RS
REFERENCES
1. Thomson JA, Itskovitz-Eldor J, Shapiro SS, et al. Embryonic stem cell lines derived from human blastocysts. Science. 1998;282:1145-1147.
2. Otani A, Dorrell MI, Kinder K, et al. Rescue of retinal degeneration by intravitreally injected adult bone marrow-derived lineage-negative hematopoietic stem cells. J Clin Invest. 2004;114:765-774.
3. Yu J, Vodyanik MA, Smuga-Otto K, et al. Induced pluripotent stem cell lines derived from human somatic cells. Science. 2007;318:1917-1920.
4. Uchida N, Buck DW, He D, et al. Direct isolation of human central nervous system stem cells. Proc Natl Acad Sci U S A. 2000;97:14720-14725.
5. Yang P, Seiler MJ, Aramant RB, Whittemore SR. In vitro isolation and expansion of human retinal progenitor cells. Exp Neurol. 2002;177:326-331.
6. Copelan EA. Hematopoietic stem-cell transplantation. N Engl J Med. 2006;354:1813-1826.
7. Brave M, Farrell A, Ching Lin S, et al. FDA review summary: Mozobil in combination with granulocyte colony-stimulating factor to mobilize hematopoietic stem cells to the peripheral blood for collection and subsequent autologous transplantation. Oncology. 2010;78:282-288.
8. Blenkinsop TA, Corneo B, Temple S, Stern JH. Ophthalmologic stem cell transplantation therapies. Regen Med. 2012;7(6 Suppl):32-39.
9. Schwartz SD, Regillo CD, Lam BL, et al. Human embryonic stem cell-derived retinal pigment epithelium in patients with age-related macular degeneration and Stargardt’s macular dystrophy: Follow-up of two open-label phase 1/2 studies. Lancet. 2015;385:509-516.
10. Song WK, Park KM, Kim HJ, et al. Treatment of macular degeneration using embryonic stem cell-derived retinal pigment epithelium: Preliminary results in Asian patients. Stem Cell Reports. 2015;4:860-872.
11. Reardon S, Cyranoski D. Japan stem-cell trial stirs envy. Nature. 2014;51:287-288.
12. Scudellari M. How iPS cells changed the world. Nature. 2016;534:310-312.
13. Researchers plan trial transplants of retinas grown from 3rd parties. The Asahi Shimbun. June 7, 2016. Available at: http://www.asahi.com/ajw/articles/AJ201606070063.html. Accessed August. 2, 2017.
14. StemCells, Inc. reports top line results for its Phase I/II study in dry age related macular degeneration [news release]. Newark, CA: StemCells, Inc., Investor Relations; June 26, 2015. http://investor.stemcellsinc.com/phoenix.zhtml?c=86230&p=RssLanding&cat=news&id=2062904. Accessed August 2, 2016.
15. Statement regarding first in-human clinical trial in inherited retinal degeneration (retinitis pigmentosa) [press release]. Boston, MA: Harvard Medical School Department of Ophthalmology. March 20, 2016 http://eye.hms.harvard.edu/news/statement-regarding-first-human-clinical-trial-inherited-retinal-degeneration-retinitis. Accessed August 2, 2016.
16. Early trial results support the safety of cell-based retinitis pigmentosa treatment [press release]. Newport Beach, CA: jCyte News; July 15, 2016 http://jcyte.com/early-trial-results-support-the-safety-of-cell-based-retinitis-pigmentosa-treatment/. Accessed August 2, 2016.
17. Otani A, Dorell MI, Kinder K, et al. Rescue of retinal degeneration by intravitreally injected adult bone marrow-derived lineage-negative hematopoietic stem cells. J Clin Invest. 2004;114:765-774.
18. Pinon RM, Pastor JC, Saornil MA, et al. Intravitreal and subretinal proliferation induced by platelet-rich plasma injection in rabbits. Curr Eye Res. 1992;11:1047-1055.
19. Park SS, Bauer G, Abedi M, et al. Intravitreal autologous bone marrow CD34+ cell therapy for ischemic and degenerative retinal disorders: Preliminary phase 1 clinical trial findings. Invest Ophthalmol Vis Sci. 2015;56:81-89.
20. Weiss JN, Levy S, Malkin A. Stem Cell Ophthalmology Treatment Study (SCOTS) for retinal and optic nerve diseases: A preliminary report. Neural Regen Res. 2015;10:982-988.
21. Weiss JN, Levy S, Benes SC. Stem Cell Ophthalmology Treatment Study (SCOTS) for retinal and optic nerve diseases: A case report of improvement in relapsing auto-immune optic neuropathy. Neural Regen Res. 2015;10:1507-1515.
22. Leung EH, Flynn HW, Jr., Albini TA, Medina CA. Retinal detachment after subretinal stem cell transplantation. Ophthalmic Surg Lasers Imaging Retina. 2016;47:600-601.
23. Kim JY, You YS, Kim SH, Kwon OW. Epiretinal membrane formation after intravitreal autologous stem cell implantation in a retinitis pigmentosa patient. Retin Cases Brief Rep. 2016. May 11, 2016 [Epub ahead of print]
24. Ramsden CM, Powner MB, Carr AJF, Smart MJK, da Crus L, Coffey PF. Stem cells in retinal regeneration: Past, present and future. Development. 2013;140:2576-2585.



