Take-home points
|
 |
|
Bios Dr. Alsoudi is an ophthalmology resident focusing on surgical retina and ocular oncology at Baylor College of Medicine, Houston. Dr. Skrehot is a medical student at Baylor College of Medicine. Dr. Rahimy is a vitreoretinal specialist at Palo Alto Medical Foundation as well as an adjunct clinical assistant professor of ophthalmology at Byers Eye Institute at Stanford University, both in Palo Alto, California. DISCLOSURES: The authors have no relevant relationships to disclose. |
Research has emerged over the past few years that has shown that geographic atrophy progresses more rapidly than previously thought.1,2 That created an opportunity for early treatment, which resulted in the Food and Drug Administration earlier this year approving pegcetacoplan (Syfovre, Apellis Pharmaceuticals) for treatment of GA and then granting priority review of avacincaptad pegol (Iveric Bio), setting an action date for August.
As more treatments emerge, understanding the natural progression of GA and the baseline characteristics that predict progression will help us to choose the optimal strategy. Here, we report on recent literature into the natural progression of GA, the rate of progression, interventions to slow its progression and the ultimate implications of untreated GA.
Natural history of GA
The advanced form of age-related macular degeneration, GA progresses at a highly variable rate. Factors including baseline lesion size, lesion location, multifocality, imaging patterns and fellow-eye status have all been implicated in predicting disease progression and outcomes.
Most of the visual loss in AMD occurs in its advanced stages, which has two typical clinical forms: GA and nAMD. GA is characterized by loss of choriocapillaris, retinal pigment epithelium and photoreceptors.3 These areas of atrophy enlarge and coalesce, creating a “geographic” lesion with sharply demarcated borders between the atrophied and normal areas of the retina.
GA usually begins parafoveally and progresses to involve the fovea. With unrelenting progression, those with unilateral GA often go on to develop lesions at a more rapid rate in the fellow eye, as early as within the first 12 months of follow-up.2
Irreversible vision loss in GA is well documented, but the relationship between GA and vision is complex because of the wide variety of lesion locations, focality and degree of foveal involvement. A 2018 review on the progression of GA didn’t find strong correlations between best-corrected visual acuity and lesion enlargement, thought to be due to the variable extent of foveal sparing.4 However, newer data from the prospective Proxima A and B clinical trials published in 2020 suggested lesion size progression is associated with a decline in vision.2
Similar levels of vision decline in patients with nonfoveal and foveal lesions have also been described, suggesting GA of any form may have devastating implications on vision.8 Despite the natural history of GA leading to significant loss of visual function over a two-year period even in patients with earlier disease states, limited therapeutic strategies exist to prevent its onset or progression.2
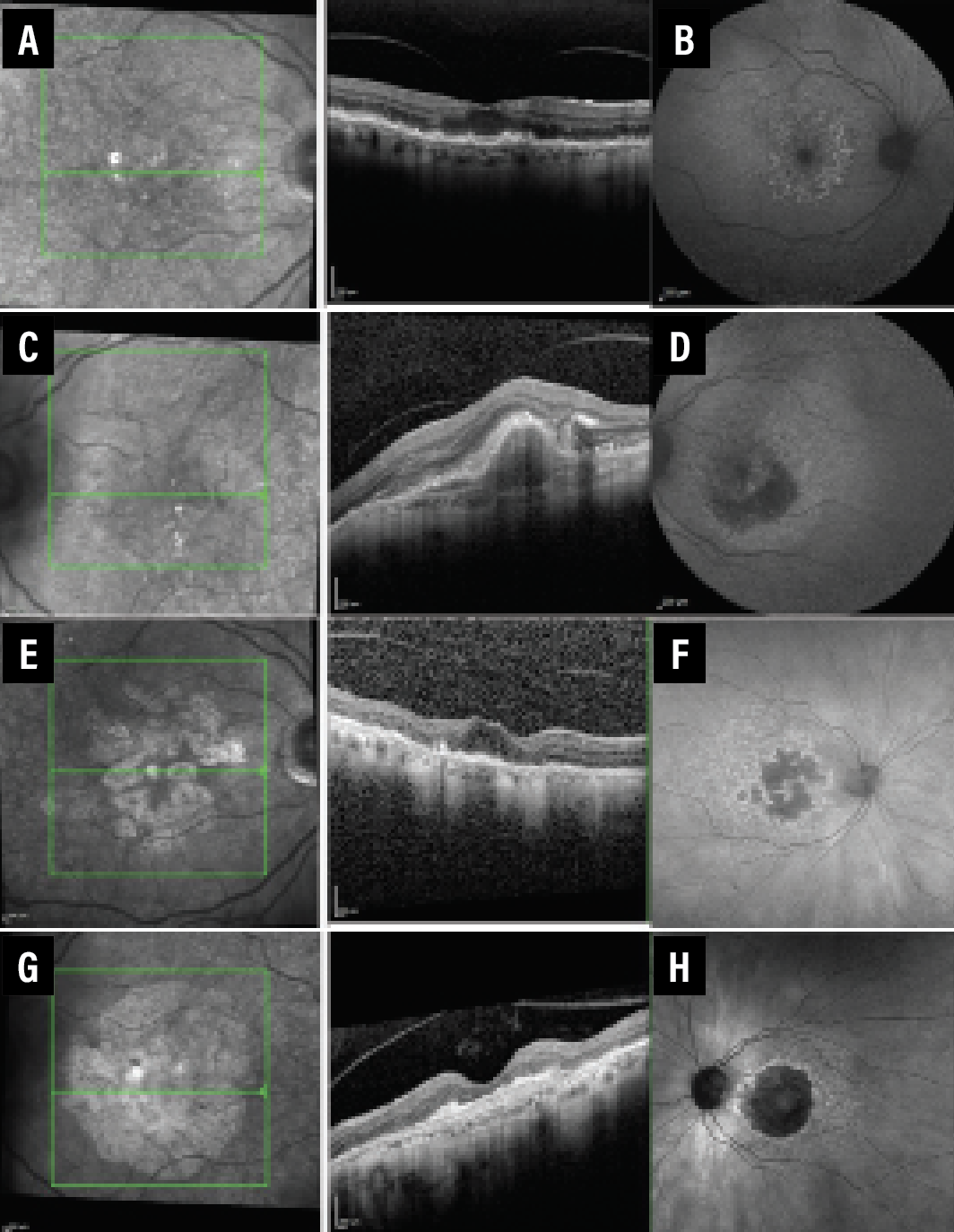 |
| Figure 1. A through D) Spectral-domain optical coherence tomography and fundus autofluorescence of a patient initially presenting with exudative age-related macular degeneration of the left eye who was subsequently treated with anti-VEGF injections. E through H) Over the ensuing three years, the fellow right eye developed multifocal geographic atrophy encroaching near the fovea with a speckled fundus autofluorescence pattern. |
In addition to lesion characteristics in the study eye, demographic factors, health status and lesion characteristics in the fellow eye have been linked with GA progression and subsequent BCVA decline. A recent large retrospective study from the United Kingdom found the type and stage of AMD in the fellow eye affected the GA progression rate in the study eye.5 It also found novel associations of age, female sex and cardiovascular disease with more rapid GA progression, whereas diabetes and glaucoma were found to decrease the risk of GA progression.5
GA progression
Color fundus photography, fundus autofluorescence and optical coherence tomography are commonly used to assess GA lesion size and other characteristics to evaluate progression risk. The rate of progression of atrophy in the setting of AMD has been reported to be 1.5 to 2.5 mm2/year, with significant variability between individuals and even within individual patients.2,4,6,7 The ability to identify GA progression early and understand high-risk signs of progression improves our understanding of the natural history of the disease, which can inform our decisions for early intervention as more treatment options become available.
Multifocal lesions, larger baseline lesion size, perilesional banded and diffuse FAF pattern as well as nonfoveal location have all been implicated in more rapid rates of GA progression (Figure 1). Moderate evidence supports more rapid rates of progression in patients with a history of a higher progression rate in the fellow eye and greater extent of abnormal hyperautofluorescence on FAF (Figure 2).4
More recently, an analysis using real-world data from the American Academy of Ophthalmology Intelligent Research In Sight (IRIS) registry highlighted a marked deterioration of vision within the two years of the study, suggesting that GA may be progressing faster than previously thought.1 This analysis also found that the time to foveal encroachment is much quicker than previous studies have reported.8 As reports emerge supporting the more rapid and progressive nature of GA, an increasing need for early intervention to slow or prevent GA progression to the fovea has become more apparent.
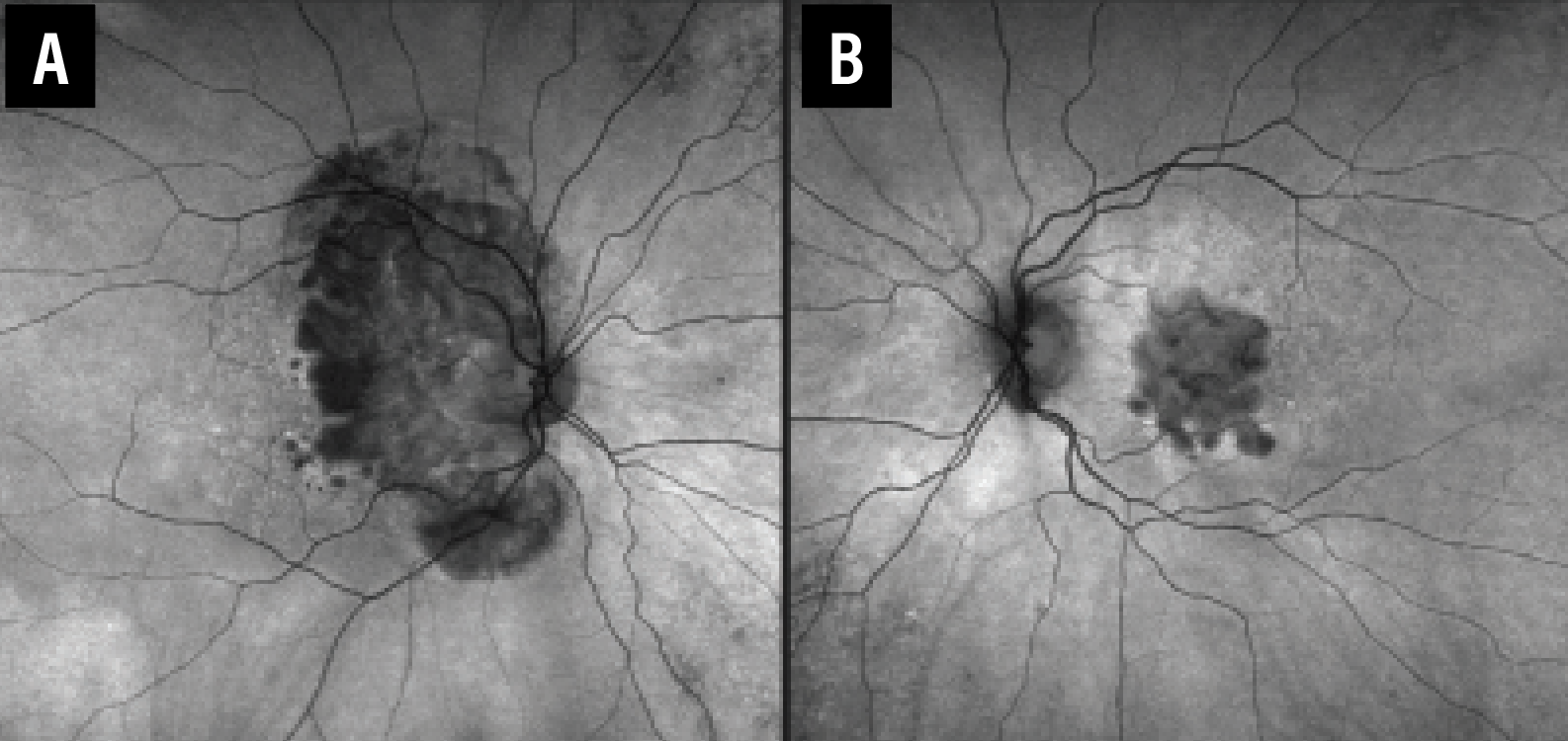 |
| Figure 2. A) Fundus autofluorescence of a patient with advanced end-stage geographic atrophy in the right eye. B) The left eye demonstrates abnormally increased hyperautofluorescence around a central foveal GA lesion. |
More recently, subretinal drusenoid deposits (SDDs), also commonly known as reticular pseudodrusen (RPD), have been identified as a macular risk feature that may aid in predicting GA progression in individuals with low or moderate AMD severity.9 RPD seemed to have a modest impact on progression. Furthermore, SDD was associated with a higher risk of progression to GA with and without other macular risk factors (Figure 3).
These studies have shown that accurate prognostic prediction of the GA progression risk requires several phenotypic and diagnostic markers. These findings support the new understanding of GA progression, its variability and the need for more information on the pathogenesis of GA to create more accurate prediction algorithms.
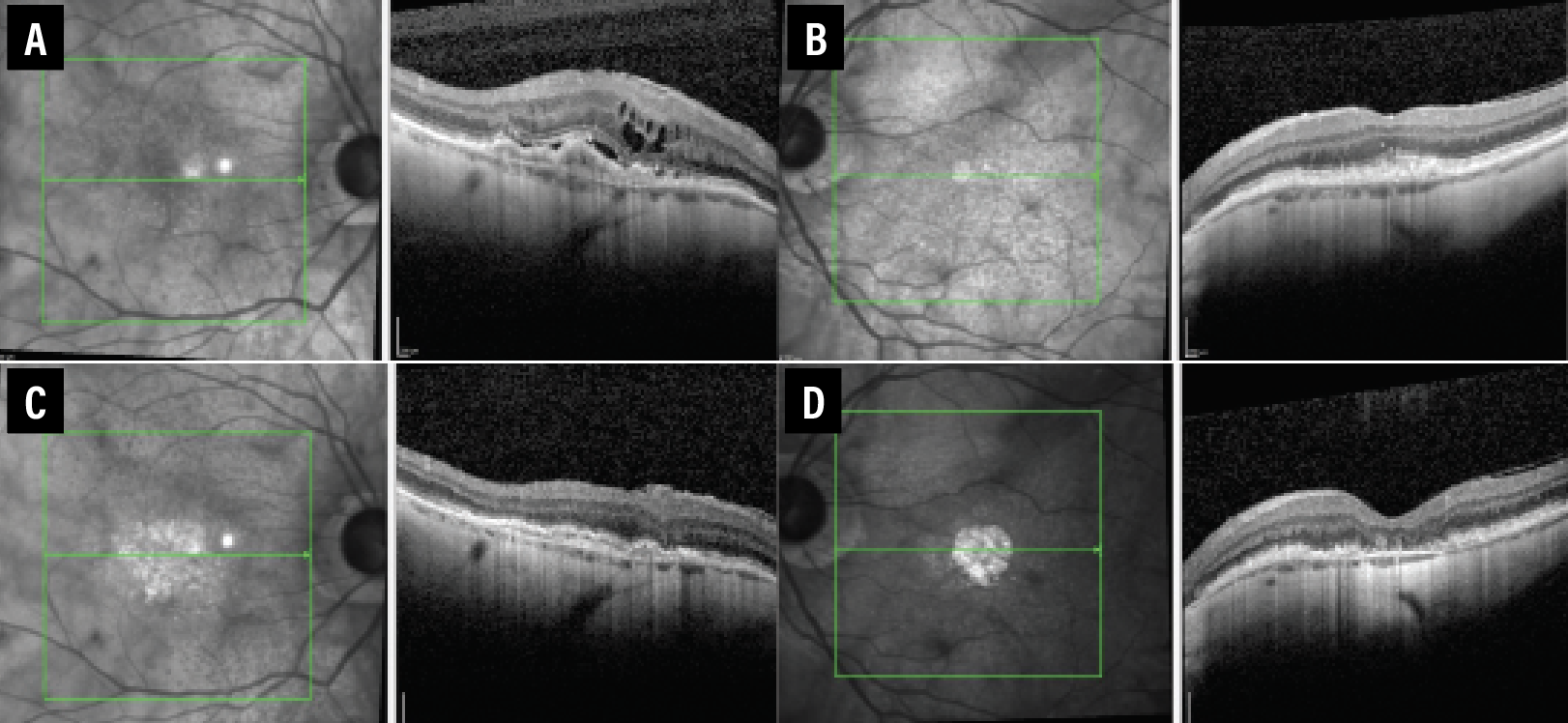 |
| Figure 3. A, B) Spectral-domain optical coherence tomography in a patient with exudative age-related macular degeneration of the right eye and nonexudative AMD of the left eye shows subretinal drusenoid deposits. C) The right eye received treatment with anti-VEGF therapy. D) The left eye subsequently developed foveal geographic atrophy. |
Interventions to slow progression
Although vascular endothelial growth factor inhibitors have been effective in treating neovascular AMD, effective GA treatments have remained an unmet need for many years. In early AMD, AREDS vitamins have demonstrated effectiveness in preventing progression to more severe AMD, but had no impact on GA specifically.10
FDA approval of pegcetacoplan came after results from the FILLY, OAKS and DERBY trials showed that the treatment reduced the rate of GA lesion growth, as measured by FAF.11
Despite GA being a multifactorial and incompletely understood disease state, more recent evidence suggested that complement cascade dysfunction plays a role in its development.12 These findings set off a race to develop complement inhibitors to target the cascade.
Pegcetacoplan, a C3 complement inhibitor that had been approved for treatment of paroxysmal nocturnal hemoglobinuria, became a front runner among other candidates when one of two large, multicenter, randomized controlled trials supported its effectiveness in slowing the progression of GA through regulating excessive activation of the complement immune system. Despite the growing evidence to support pegcetacoplan in slowing GA growth and the exciting implications of these findings, treatment hasn’t demonstrated improvement in visual function.
However, the most recent results have suggested lower vision loss and better quality of life compared to sham in a post hoc analysis.13 While the safety profile of pegcetacoplan is acceptable, an increased risk of developing exudative AMD in treated patients has been described.
If the prospect of pegcetacoplan wasn’t already exciting enough, another agent, intravitreal avacincaptad, may be FDA-approved by the fall. Avacincaptad is a C5 complement inhibitor that demonstrated a statistically significant reduction in GA progression with a favorable safety profile, as described in the GATHER1 and GATHER2 trials.14
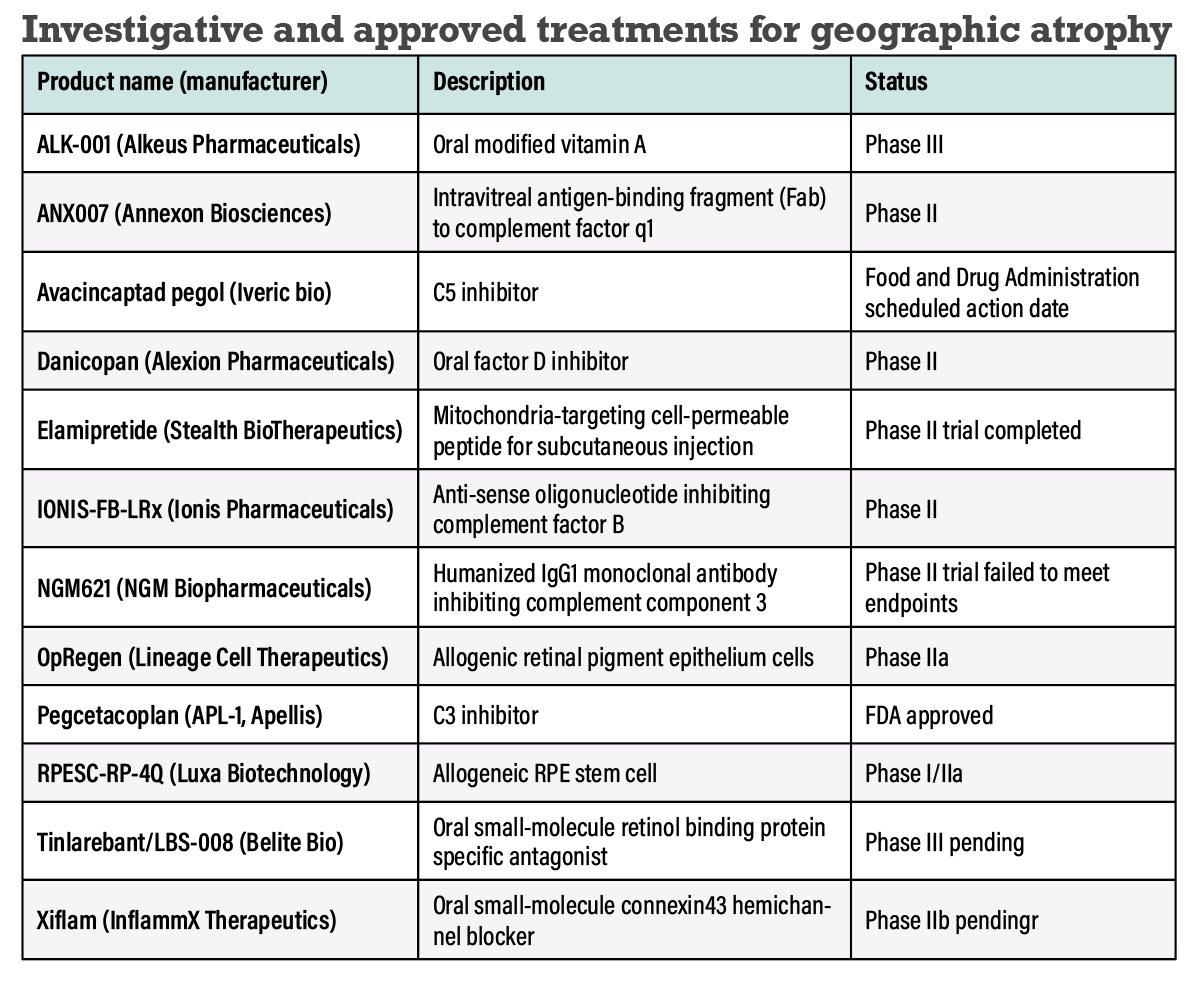
A robust pipeline of other drugs in earlier stages of development has shown promise (Table). These drugs mark the beginning of an effort to manage GA in ways that previously weren’t possible.
 |
Implications of untreated GA
The prevalence of GA increases with advancing age and it’s a leading cause of blindness in the elderly. Without treatment, GA will inevitably progress in eyes that have it and in fellow eyes at a more rapid rate.2 Patients with GA may experience difficulty with vision-related quality-of-life tasks, such as reading, driving and working. The economic burden through direct costs for treatment and indirect costs through lost productivity are substantial and expected to continue to grow without interventions to slow the progress of GA.15
Untreated GA can also have significant psychological and social impacts on patients. Visual impairment can lead to depression, social isolation and decreased mobility, reducing overall quality of life and increasing the risk of falls and other injuries.15
Even more demoralizing for these patients has been the lack of treatment options to reverse and slow the disease’s natural course. As a leading cause of blindness in the elderly in the western world and with immense negative impacts on quality of life, any validated treatment options for GA should be celebrated, even if the progress is measured in incremental steps rather than a major leap forward. While pegcetacoplan, by slowing the growth of lesions, gives new hope to patients with GA, research continues toward future therapies that can hopefully improve visual function or even prevent the onset of GA in the longer-term race against this disease.
Bottom line
Ultimately, the new understanding of the rapid, progressive nature of GA coupled with findings to support early treatment options has provided a glimmer of hope, but still leaves much to be desired in the effort to develop additional treatment strategies to slow or even prevent GA progression. Pegcetacoplan is an important first step in treatment for patients with advanced atrophic AMD. A discussion with our patients who have advanced atrophic AMD should address the need for repeated treatments, drug efficacy and safety signals. RS
REFERENCES
1. Rahimy E, Khan MA, Ho AC, et al. Progression of geographic atrophy: Retrospective analysis of patients from the IRIS Registry. Ophthalmol Sci. Published online April 19, 2023:100318. doi:10.1016/j.xops.2023.100318.
2. Holekamp N, Wykoff CC, Schmitz-Valckenberg S, et al. Natural history of geographic atrophy secondary to age-related macular degeneration: results from the prospective Proxima A and B clinical trials. Ophthalmology. 2020;127:769-783.
3. Sarks JP, Sarks SH, Killingsworth MC. Evolution of geographic atrophy of the retinal pigment epithelium. Eye (Lond). 1988;2:552-577.
4. Fleckenstein M, Mitchell P, Freund KB, et al. The progression of geographic atrophy secondary to age-related macular degeneration. Ophthalmology. 2018;125:369-390.
5. Chakravarthy U, Bailey CC, Scanlon PH, et al. Progression from early/intermediate to advanced forms of age-related macular degeneration in a large UK Cohort: Rates and risk factors. Ophthalmol Retina. 2020;4:662-672.
6. Holz FG, Bindewald-Wittich A, Fleckenstein M, et al. Progression of geographic atrophy and impact of fundus autofluorescence patterns in age-related macular degeneration. Am J Ophthalmol. 2007;143:463-472.
7. Sunness JS, Margalit E, Srikumaran D, et al. The long-term natural history of geographic atrophy from age-related macular degeneration: enlargement of atrophy and implications for interventional clinical trials. Ophthalmology. 2007;114:271-277.
8. Lindblad AS, Lloyd PC, Clemons TE, et al. Change in area of geographic atrophy in the Age-Related Eye Disease Study: AREDS report number 26. Arch Ophthalmol. 2009;127:1168-1174.
9. Agrón E, Domalpally A, Cukras CA, et al. Reticular pseudodrusen: The third macular risk feature for progression to late age-related macular degeneration: Age-Related Eye Disease Study 2 Report 30. Ophthalmology. 2022;129:1107-1119.
10. Chew EY, Clemons TE, Agrón E, et al. Long-term outcomes of adding lutein/zeaxanthin and ω-3 fatty acids to the AREDS supplements on age-related macular degeneration progression: AREDS2 Report 28. JAMA Ophthalmol. 2022;140:692-698.
11. Nittala MG, Metlapally R, Ip M, et al. Association of pegcetacoplan with progression of incomplete retinal pigment epithelium and outer retinal atrophy in age-related macular degeneration: A post hoc analysis of the FILLY randomized clinical trial. JAMA Ophthalmol. 2022;140:243-249.
12. Boyer DS, Schmidt-Erfurth U, van Lookeren Campagne M, Henry EC, Brittain C. The pathophysiology of geographic atrophy secondary to age-related macular degeneration and the complement pathway as a therapeutic target. Retina. 2017;37:819-835.
13. Apellis presents Phase 3 functional analyses of SyfovreTM (pegcetacoplan injection) for geographic atrophy [press release. Waltham, MA.; Apellis Pharmaceuticals; May 28, 2023. https://investors.apellis.com/news-releases/news-release-details/apellis-presents-phase-3-functional-analyses-syfovretm.
14. Jaffe GJ, Westby K, Csaky KG, et al. C5 Inhibitor avacincaptad pegol for geographic atrophy due to age-related macular degeneration: A randomized pivotal Phase 2/3 trial. Ophthalmology. 2021;128:576-586.
15. Sarda SP, Heyes A, Bektas M, et al. Humanistic and economic burden of geographic atrophy: A systematic literature review. Clin Ophthalmol. 2021;15:4629-4644.



