 |
 |
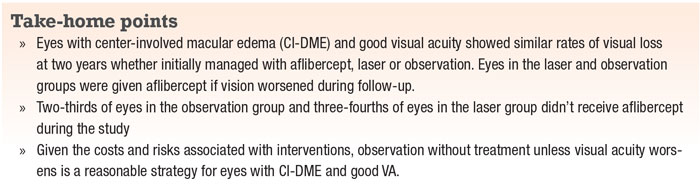
Center-involved macular edema with good vision is a clinical scenario many retina specialists and general ophthalmologists commonly see (Figure 1).1 However, until recently, the best strategy for managing such patients was unknown.
Before the 2019 publication of the DRCR Retina Network Protocol V, some ophthalmologists treated eyes presenting with central fluid on optical coherence tomography because they worried about the potential for long-term damage that might eventually lead to visual loss. Other ophthalmologists worried about treating patients with excellent vision and minimal symptoms with an invasive ocular procedure despite the inherent risks, such as endophthalmitis.2
Laying the foundation for Protocol V
It’s not surprising that good visual acuity and central edema can co-exist given the poor correlation between OCT central subfield thickness (CST) and vision.3 In the Early Treatment Diabetic Retinopathy Study, a large percentage of eyes at baseline had VA of 20/25 or better; 27 percent in the focal/grid laser group and 40 percent in the observation group.4 In both groups, only a few eyes lost 5 letters or more at two years of follow-up (40 percent in the observation group and 25 percent in the laser group).
Furthermore, not all fluid results in visual acuity loss, as evidenced by a substantial percentage of eyes with persistent DME in DRCR Retina Network Protocols I and T that maintained vision despite chronic persistent fluid.5, 6
Protocol V was a multicenter randomized clinical trial of the DRCR Retina Network that aimed to answer the question of how to best manage eyes with good vision despite CI-DME.7 The study compared three distinct initial management strategies: intravitreal aflibercept (Eylea, Regeneron Pharmaceuticals); macular focal/grid photocoagulation; and observation.
Two strategies, observation and laser, involved close follow-up with clearly defined criteria for initiating anti-VEGF therapy if VA declined consistently or substantially.
In eyes with CI-DME and good VA, Protocol V reported no difference in VA loss at two years regardless of treatment strategy. Given the potential complications and costs associated with either anti-VEGF injections or focal/grid laser, observation without treatment unless VA worsens may be a reasonable, cost-effective and safe strategy. Here, we review the clinical implications of the key findings of Protocol V.
 |
Treatment protocol
The study had three groups: aflibercept (n=226); observation with aflibercept pro re nata for vision loss during follow-up (n=236); and laser with aflibercept p.r.n. for vision loss during follow-up (n=240). The study had a high completion rate of 92 percent (excluding deaths) at two years.
All patients in the aflibercept group received treatment at baseline and were re-evaluated at each visit for possible re-injection. Injections were continued if the VA worsened or improved by 5 letters or more or if OCT CST changed by 10 percent or more from either of the previous two injections compared to the current visit. Injections were deferred if VA and CST were stable for two consecutive visits and either 24 weeks had passed since injections were started or if VA was 20/20 or better and CST on OCT was below machine- and gender-based thresholds used in previous studies to detect DME.
Laser group patients received laser photocoagulation (focal/modified grid) at baseline. The observation group received no treatment initially. Both groups received aflibercept if VA decreased more than 10 letters from baseline (approximately 2 lines) at one visit or by 5 to 9 letters (1 to 2 lines) at two consecutive visits. While OCT changes were used to modify follow-up durations, anatomic changes in retinal thickness didn’t determine the initiation of treatment.
 |
Similar outcomes across groups
The primary study outcome was a loss of 5 letters or more (approximately 1 line) at two years. The percentage of eyes that met that outcome didn’t differ significantly between the treatment groups: 16, 17 and 19 percent in the aflibercept, laser and observation groups, respectively.
At two years, the mean VA letter score change from baseline also didn’t differ statistically between the groups: +0.9, +0.1 and -0.4 letters. Mean VA at two years was equivalent to 20/20 in each treatment group. In addition, the groups didn’t differ significantly in number of eyes with a 5-
letter or more vision loss or gain or a 10-letter or more loss at two years.
Perhaps the only notable difference between the groups was the number of eyes with a VA of 20/20 or better at two years: 77 percent for the aflibercept group vs. 66 percent for observation (p=0.03). However, a similar percentage of eyes (84 to 86 percent) in each of the three groups had a VA of 20/25 or better at two years.
Although the mean one-year change in OCT was significantly greater in the aflibercept group, this difference all but vanished at two years. Aflibercept patients had a rapid decrease in CST by eight weeks, which remained stable until the two-year mark. This contrasts with the observation and laser groups, which had a slower but steady decrease in CST from baseline at two years.
When aflibercept was initiated
Aflibercept was initiated for vision loss in about a quarter of the eyes in the laser group and about a third in the observation group. The cumulative probability for initiating aflibercept was higher in the observation group than the laser group at both one and two years. The median number of aflibercept injections over two years was seven and nine in the laser and observation groups, respectively, vs. eight in the aflibercept group.
Other observation group outcomes
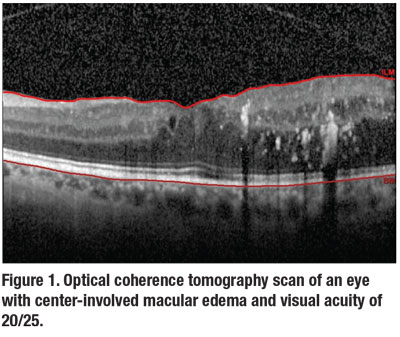
Most eyes initially managed with observation didn’t require aflibercept (66 percent, Figure 2). Median VA in this group was 20/20 and 31 percent had spontaneous resolution of DME at two years.8 Among observation eyes receiving aflibercept, median two-year VA was 20/25, and 70 percent lost less than 1 line of vision.
In the observation group, 19 percent of eyes experienced a 10-letter or more loss at least once for which treatment was initiated.8 In total, 39 percent of observation eyes had a 5-to-9-letter loss at least once, of which only 32 percent had sustained VA loss on the subsequent visit and required initiation of aflibercept treatment.8
In other words, 68 percent of eyes showing an initial 5-to-9-letter loss didn’t require treatment on the subsequent visit, demonstrating the month-to-month variability in VA seen in patients with DME.
Greater OCT thickness at baseline, worse diabetic retinopathy severity (moderately severe nonproliferative DR or worse) and recent (within four months) DME treatment in the non-study eye were all associated with use of aflibercept in the observation group. Age, gender, ethnicity and baseline HbA1c were not associated with an increased probability of receiving aflibercept.
 |
One approach as good as the other
The Protocol V results demonstrate that visual outcomes are good with all three initial management strategies of aflibercept, macular laser and observation. However, beginning immediate anti-VEGF therapy in eyes with CI-DME and good vision doesn’t seem to derive any additional benefit. In fact, most eyes in both the laser (75 percent) and observation groups (66 percent) had stable vision and didn’t require rescue aflibercept therapy.
It’s important to highlight that the observation and laser protocols were only the initial strategies adopted. Patients were required to undergo frequent follow-ups and assessments to determine whether their VA was stable or worsening. The study employed a vision-based algorithm to determine when aflibercept treatment was initiated in the laser and observation groups. Hence the treatment of those groups wasn’t with monotherapy, but reflected a strategy of laser or observation for patients who remained stable from baseline and rescue aflibercept therapy for those whose vision worsened over time. Although OCT worsening was used as a parameter to determine follow-up durations, it wasn’t used to determine anti-VEGF treatment initiation.
We must carefully weigh the option of starting therapy vs. a more conservative approach. Aflibercept injections are costly and carry a risk, albeit small, of complications such as endophthalmitis ( less than 0.1 percent).9 Given that observation with p.r.n. aflibercept achieves outcomes similar to immediate aflibercept injections, initial observation in eyes with center-involved DME and good vision seems reasonable. RS
REFERENCES
1. Bressler NM, Varma R, Doan QV, et al. Underuse of the health care system by persons with diabetes mellitus and diabetic macular edema in the United States. JAMA Ophthalmol. 2014;132:168-173.
2. Kiss S, Dugel PU, Khanani AM, et al. Endophthalmitis rates among patients receiving intravitreal anti-VEGF injections: a USA claims analysis. Clin Ophthalmol. 2018;12:1625-1635.
3. Bressler NM, Odia I, Maguire M, et al. Association between change in visual acuity and change in central subfield thickness during treatment of diabetic macular edema in participants randomized to aflibercept, bevacizumab, or ranibizumab: a post hoc analysis of the Protocol T randomized clinical trial. JAMA Ophthalmol. 2019;137:977-985.
4. Early photocoagulation for diabetic retinopathy. ETDRS Report Number 9. Early Treatment Diabetic Retinopathy Study Research Group. Ophthalmology. 1991;98:766-785.
5. Bressler NM, Beaulieu WT, Glassman AR, et al. Persistent macular thickening following intravitreous aflibercept, bevacizumab, or ranibizumab for central-involved diabetic macular edema with vision impairment: a secondary analysis of a randomized clinical trial. JAMA Ophthalmol. 2018;136:257-269.
6. Bressler SB, Ayala AR, Bressler NM, et al. Persistent macular thickening after ranibizumab treatment for diabetic macular edema with vision impairment. JAMA Ophthalmol. 2016;134:278-285.
7. Baker CW, Glassman AR, Beaulieu WT, et al. Effect of initial management with aflibercept vs laser photocoagulation vs observation on vision loss among patients with diabetic macular edema involving the center of the macula and good visual acuity: a randomized clinical trial. JAMA. 2019;321:1880-1894.
8. Glassman AR, Baker CW, Beaulieu WT, et al. Assessment of the DRCR Retina Network approach to management with initial observation for eyes with center-involved diabetic macular edema and good visual acuity: a secondary analysis of a randomized clinical trial. JAMA Ophthalmol. 2020;138:341-349.
9. Bhavsar AR, Glassman AR, Stockdale CR, Jampol LM. Elimination of topical antibiotics for intravitreous injections and the importance of using povidone-iodine: Update from the Diabetic Retinopathy Clinical Research Network. JAMA Ophthalmol. 2016;134:1181-1183.



