Take-home Points
|
 |
| Bios
|
Ocular gene therapy is emerging as a potentially disruptive technology in the management of inherited and acquired retinal conditions.1-4 The term gene therapy broadly encompasses gene replacement strategies to replace nonfunctional genes with normal copies in inherited retinal diseases, biofactory approaches to generate soluble proteins such as anti-VEGF biologic agents for age-related macular degeneration and emerging gene-editing tools to modify gene expression at the DNA level.2-4
The mainstay of these therapies involves intraocular delivery of viral vectors that can carry the therapeutic gene into the nuclei of target cells. The choice of vector and route of administration are critical determinants of the efficacy and safety of these delivery platforms, and recent advances are broadening the range of options available.
Delivery vectors
Viral vectors leverage the natural ability of these microorganisms to infect and transport genetic material into host cells. The effectiveness of a gene-delivery vector is determined by high expressivity, large transgene-carrying capacity, long durability, low immunogenicity and low risk of mutagenicity.
Adenoviruses were among the first used for gene therapy due to their high infectivity and large capacity, but they’ve largely been abandoned due to their strong immunogenicity and short duration of expression.5
Lentiviruses are a subtype of retroviruses including HIV that also have high infectivity and a large carrying capacity. However, because they naturally integrate into host genomes, they carry an increased risk of mutation and oncogenesis.6
Adeno-associated viruses emerge
By contrast, adeno-associated viruses (AAVs) are the most common viral vector used for ocular gene therapy due to their ability to transduce multiple retinal cell types, low pathogenicity and low risk of mutations because they don’t integrate into the host genome.7
Different serotypes of AAV capsids provide different tropisms for different retinal cell types. For example, intravitreal AAV2 and AAV8 can target retinal ganglion cells, while AAV2, AAV5, AAV7, AAV8 and AAV9 can transduce photoreceptors and RPE cells when given subretinally.7
The first Food and Drug Administration-approved ocular gene therapy, voretigene neparvovec-rzyl (Luxturna, Spark Therapeutics), uses an AAV2 vector to deliver a functional copy of the RPE65 gene for the treatment of Leber congenital amaurosis type 2.8 Other retinal conditions for which AAV gene therapies are under investigation include choroideremia, achromatopsia, X-linked retinitis pigmentosa, X-linked retinoschisis and AMD.
Expanding use of viral vectors
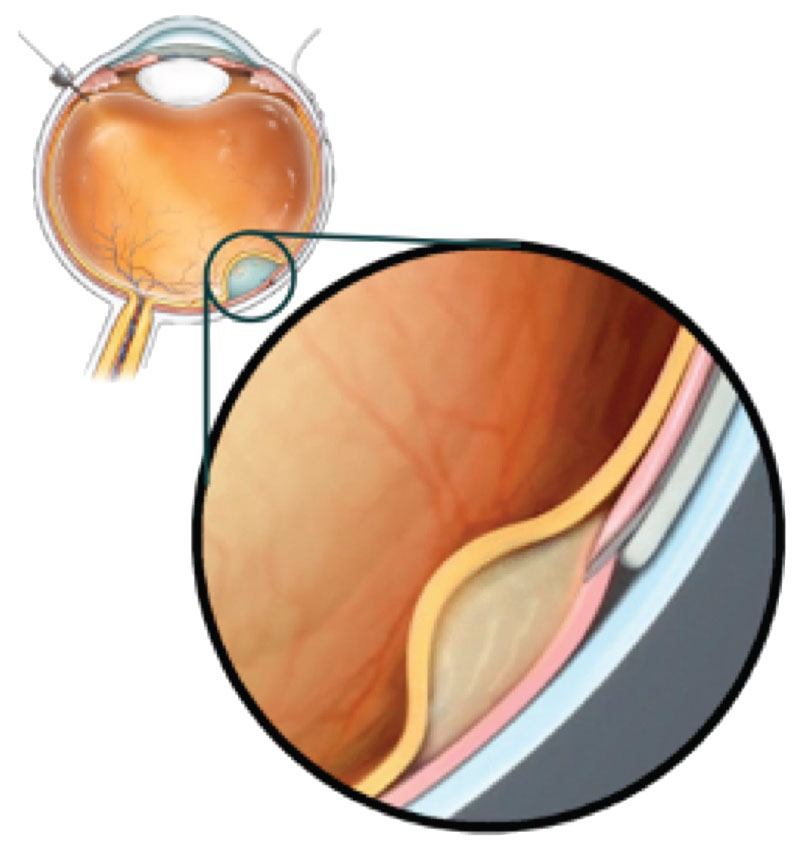 |
| Figure 1. A subretinal delivery system using a microneedle deployed from a suprachoroidal catheter can access the subretinal space without creating a retinotomy. (Courtesy Gyroscope Therapeutics) |
New advances are also being investigated to expand the use of viral vectors. AAVs have a limited capacity and can only carry genes smaller than 4.7kb. Therefore, larger genes, such as the ABCA4 gene (6.8kb) in Stargardt disease, must rely on lentivirus or nonviral vectors for effective delivery.9
To overcome this limitation, larger genes could be split in half and delivered with dual AAV vectors, then reconstituted in host cells by exploiting natural mechanisms of rejoining DNA such as splicing or homologous recombination.10 Efforts are also ongoing to engineer non-integrating lentiviruses to minimize the risk of mutagenesis.11
Finally, newer generations of AAV have been designed to overcome natural ocular barriers. Typically, AAV vectors for retinal gene therapies must be delivered by subretinal injections because viral particles in the vitreous cavity are prevented from reaching photoreceptors and retinal pigment epithelium by the internal limiting membrane.
New AAV capsids such as the AAV2.7m8 have been developed using methods known as in vitro “directed evolution” which can overcome the ILM barrier and transduce the retina, even when they’re administered intravitreally.12 The Phase I OPTIC Trial is evaluating intravitreal ADVM-022 (Adverum Biotechnologies), an AAV2.7m8 to deliver aflibercept for treatment of anti-VEGF-responsive neovascular AMD (ClinicalTrials.gov NCT03748784).
Non-viral delivery vectors
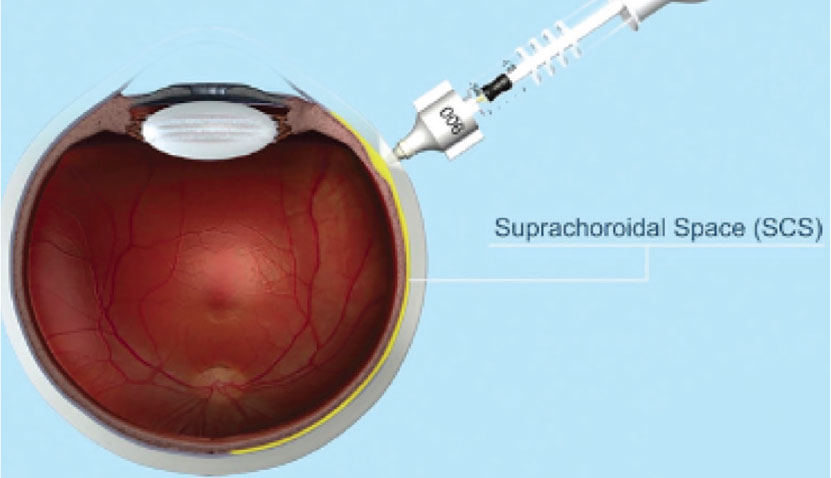 |
| Figure 2. A transscleral microneedle can be used to deliver drugs or viral vectors to the suprachoroidal space. (Courtesy Clearside Biomedical) |
Non-viral delivery vectors are nonpathogenic and less likely to be immunogenic or mutagenic. They can be engineered with a very large carrying capacity (20kb), but are also generally less effective and less durable for gene transduction. Emerging nonviral technologies include synthetic polymers and nanoparticles, lipid-based delivery systems and cell penetrating peptides.13
DNA nanoparticles have been evaluated in early clinical trials for cystic fibrosis, and in preclinical studies for retinitis pigmentosa, LCA and Stargardt disease in mouse and rabbit models,13-15 with studies demonstrating stable expression up to two years in mice.14
Some portions of the eye may also be amenable to electroporation—the use of an electric field to create temporary pores in cell membranes to allow DNA uptake. Electroporation of DNA plasmids into ciliary muscles can serve as a bio-factory for therapeutic proteins (Eyevensys) and appears effective in animal models of uveitis, RP and wet AMD for up to six months.16 This platform is undergoing clinical trials for non-infectious uveitis (ClinicalTrials.gov NCT03308045).
Delivery methods
 |
Unlike intravitreal injections, which can be readily performed by retina specialists in office settings, subretinal injections require vitreoretinal surgery and are susceptible to complications associated with vitrectomy and creation of a subretinal bleb, especially in eyes with IRDs where retinal tissues may be more atrophic and fragile. In one of the gene therapy trials for choroideremia, a patient developed intraretinal and subretinal hemorrhage during the injection procedure, resulting in localized atrophy and severe vision loss.17
Strategies to mitigate these surgical complications include the use of a saline “pre-bleb” to confirm successful size, location, and retinal elevation of the therapeutic region; automated viscous fluid injection rather than manual depression of the syringe plunger; and intraoperative optical coherence tomography (iOCT) for real-time visualization. More recent clinical trials for choroideremia that use iOCT have reported fewer complications than earlier studies.18
Other anecdotal surgical pearls include use of a subretinal air bubble, air tamponade and supine postoperative positioning.
The most effective route
Despite the potential surgical complications, subretinal injections remain the most effective route for transducing the outer retina as it overcomes the ILM barrier. Another major advantage is the immune privilege of the subretinal space.19,20 Preclinical animal studies and human trials have reported significantly higher rates of ocular inflammation after intravitreal compared with subretinal AAV injections.19
It appears that AAV in the vitreous cavity readily escapes into circulation through trabecular outflow, prompting a host immune response that can trigger both intraocular inflammation and adaptive immunity against future AAV delivery, which have been reported in patients who require a second eye treatment.21-23
Cases of intraocular inflammation after subretinal gene therapies are believed to arise from reflux or leakage of virus into the vitreous. This concern may be overcome with an innovative subretinal delivery system (Orbit SDS, Gyroscope Therapeutics) that uses a flexible canula inserted through the suprachoroidal space, accessed via a surgical sclerotomy (Figure 1). This system can deploy a microneedle that punctures through the choroid to access the subretinal space for vector delivery, thus avoiding the vitrectomy and retinotomy required for transvitreal subretinal delivery.
The ongoing FOCUS phase I/II clinical trial aims to evaluate an investigational AAV-based complement factor I (CFI) gene therapy (GT005) administered with this system in patients with geographic atrophy, and will enable a comparison between subretinal delivery and transvitreal methods (ClinicalTrials.gov NCT03846193).24,25
Suprachoroidal injection
 |
A novel ocular gene delivery route involves accessing the suprachoroidal space. Suprachoroidal injection of a triamcinolone acetonide suspension using trans-scleral microneedles has demonstrated safety and efficacy for macular edema due to noninfectious uveitis in a Phase III trial (Figure 2).26 The injections enabled targeted delivery of drug to the posterior pole while minimizing adverse effects on anterior segment tissue.
Like intravitreal injections, this method can be given in an office setting. Suprachoroidal delivery of AAV in pigs and nonhuman primates enabled widespread gene expression in the posterior pole, although the cellular tropism and pattern of expression differed between studies, including those from our own group.27
Due to its location outside the blood-retinal barrier, suprachoroidal AAV delivery has a potential risk of immunogenicity, although our studies employed a green fluorescent protein transgene that’s foreign to primates.28 Suprachoroidal injections of an AAV that expresses a native or humanized protein should reduce the risk of inflammation, as shown in new Phase I/II studies evaluating suprachoroidal delivery of RGX-314 (RegenXbio), an AAV8 encoding a ranibizumab-like anti-VEGF protein for neovascular AMD (ClinicalTrials.gov NCT04567550).
Suprachoroidal delivery of DNA nanoparticles is also under preclinical investigation and could minimize the immunogenicity associated with viral vectors.15,29
Bottom line
Ocular gene therapy is a promising and emerging field with the potential to treat both rare IRDs and more common acquired retinal conditions. Newer generations of viral and synthetic vectors may improve expressivity and carrying capacity while reducing immunogenicity and mutagenicity. Advances in surgical and delivery methods are improving the ease of vector administration and biodistribution of gene expression. The choice of vector and route of delivery depends on the disease and transgene, as well as target location and cell types.
Gene replacement or editing therapies for IRDs may require more accurate vector delivery to specific cell types through subretinal injections, while biofactory approaches may benefit from easier, less invasive strategies to rival existing therapies. A number of exciting ongoing clinical trials will provide important insight into the potential of these novel technologies to widen the doors for ocular gene therapy. RS
REFERENCES
1. Benati D, Patrizi C, Recchia A. Gene editing prospects for treating inherited retinal diseases. J Med Genet. 2020;57:437-444.
2. Bordet T, Behar-Cohen F. Ocular gene therapies in clinical practice: viral vectors and nonviral alternatives. Drug Discov Today. 2019;24:1685-1693.
3. Moore NA, Morral N, Ciulla TA, Bracha P. Gene therapy for inherited retinal and optic nerve degenerations. Expert Opin Biol Ther. 2018;18:37-49.
4. Chung SH, Frick SL, Yiu G. Targeting vascular endothelial growth factor using retinal gene therapy. Ann Transl Med 2020. doi:10.21037/atm-20-4417
5. Lee CS, Bishop ES, Zhang R, et al. Adenovirus-mediated gene delivery: Potential applications for gene and cell-based therapies in the new era of personalized medicine. Genes Dis. 2017;4:43-63.
6. Milone MC, O'Doherty U. Clinical use of lentiviral vectors. Leukemia. 2018;32:1529-1541.
7. Wang D, Tai PWL, Gao G. Adeno-associated virus vector as a platform for gene therapy delivery. Nat Rev Drug Discov. 2019;18:358-378.
8. Miraldi Utz V, Coussa RG, Antaki F, Traboulsi EI. Gene therapy for RPE65-related retinal disease. Ophthalmic Genet. 2018;39:671-677.
9. Auricchio A, Trapani I, Allikmets R. Gene therapy of ABCA4-associated diseases. Cold Spring Harb Perspect Med. 2015;5:a017301.
10. Trapani I, Colella P, Sommella A, et al. Effective delivery of large genes to the retina by dual AAV vectors. EMBO Mol Med. 2014;6:194-211. doi:10.1002/emmm.201302948
11. Hamilton AM, Foster PJ, Ronald JA. Evaluating nonintegrating lentiviruses as safe vectors for noninvasive reporter-based molecular imaging of multipotent mesenchymal stem cells. Hum Gene Ther. 2018;29:1213-1225.
12. Dalkara D, Byrne LC, Klimczak RR, et al. In vivo-directed evolution of a new adeno-associated virus for therapeutic outer retinal gene delivery from the vitreous. Sci Transl Med. 2013;5:189ra76.
13. Adijanto J, Naash MI. Nanoparticle-based technologies for retinal gene therapy. Eur J Pharm Biopharm. 2015;95:353-367.
14. Han Z, Conley SM, Makkia R, Guo J, Cooper MJ, Naash MI. Comparative analysis of DNA nanoparticles and AAVs for ocular gene delivery. PLoS One. 2012;7:e52189.
15. Kansara VS, Cooper M, Sesenoglu-Laird O, Muya L, Moen R, Ciulla TA. Suprachoroidally delivered DNA nanoparticles transfect retina and retinal pigment epithelium/choroid in rabbits. Transl Vis Sci Technol. 2020;9:21.
16. Bigot K, Gondouin P, Bénard R, et al. Transferrin non-viral gene therapy for treatment of retinal degeneration. Pharmaceutics. 2020;12:836.
17. Dimopoulos IS, Hoang SC, Radziwon A, et al. Two-year results after aav2-mediated gene therapy for choroideremia: The Alberta experience. Am J Ophthalmol. 2018;193:130-142.
18. Lam BL, Davis JL, Gregori NZ, et al. Choroideremia gene therapy Phase 2 clinical trial: 24-month results. Am J Ophthalmol. 2019;197:65-73.
19. Nuzbrokh Y, Kassotis AS, Ragi SD, Jauregui R, Tsang SH. Treatment-emergent adverse events in gene therapy trials for inherited retinal diseases: A narrative review. Ophthalmol Ther. 2020;9:709-724.
20. Yiu G, Chung SH, Mollhoff IN, et al. Suprachoroidal and subretinal injections of AAV using transscleral microneedles for retinal gene delivery in nonhuman primates. Mol Ther Methods Clin Dev. 2020;16:179-191.
21. Seitz IP, Michalakis S, Wilhelm B, et al. Superior retinal gene transfer and biodistribution profile of subretinal versus intravitreal delivery of AAV8 in nonhuman primates. Invest Ophthalmol Vis Sci. 2017;58:5792-5801.
22. Reichel FF, Peters T, Wilhelm B, et al. Humoral immune response after intravitreal but not after subretinal AAV8 in primates and patients. Invest Ophthalmol Vis Sci. 2018;59:1910-1915.
23. Kotterman MA, Yin L, Strazzeri JM, Flannery JG, Merigan WH, Schaffer DV. Antibody neutralization poses a barrier to intravitreal adeno-associated viral vector gene delivery to non-human primates. Gene Ther. 2015;22:116-126.
24. de Smet MD, Lynch JL, Dejneka NS, Keane M, Khan IJ. A subretinal cell delivery method via suprachoroidal access in minipigs: Safety and surgical outcomes. Invest Ophthalmol Vis Sci. 2018;59:311-320.
25. Heier JS, Ho AC, Samuel MA, et al. Safety and efficacy of subretinally administered palucorcel for geographic atrophy of age-related macular degeneration: Phase 2b study. Ophthalmol Retina. 2020;4:384-393.
26. Yeh S, Khurana RN, Shah M, et al. Efficacy and safety of suprachoroidal CLS-TA for macular edema secondary to noninfectious uveitis: Phase 3 randomized trial. Ophthalmology. 2020;127:948-955.
27. Ding K, Shen J, Hafiz Z, et al. AAV8-vectored suprachoroidal gene transfer produces widespread ocular transgene expression. J Clin Invest. 2019;129:4901-4911.
28. Chung SH, Mollhoff IN, Mishra A, et al. Host immune responses after suprachoroidal delivery of AAV8 in nonhuman primate eyes. Hum Gene Ther. Published online January 15, 2021.
29. Kansara V, Muya L, Wan CR, Ciulla TA. Suprachoroidal delivery of viral and nonviral gene therapy for retinal diseases. J Ocul Pharmacol Ther. 2020;36:384-392.



