 |
Ocular toxoplasmosis, the most common cause of posterior uveitis,1-3 is a manifestation of infection from the intracellular protozoan Toxoplasma gondii. Infection is often acquired through ingestion of contaminated water or food. Transplacental transmission infecting the fetus has also been reported.1-5
Classical clinical presentation
The usual presenting symptoms of ocular toxoplasmosis are floaters and blurred vision. Clinical findings are actually the result of inflammation due to reactivation of the infection that occurred in utero or after birth. The retina is the most common ocular site for infection, but the underlying choroid can become inflamed secondarily, leading to retinochoroiditis.
The classic presentation of toxoplasmosis retinochoroiditis (TRC) is a fluffy white nidus of retinitis often adjacent to a pigmented chorioretinal scar, with overlying vitritis, producing the classic “headlight in the fog” finding.1,5 Lesion size and thickness can dictate the amount of vitreous haze and cell.6
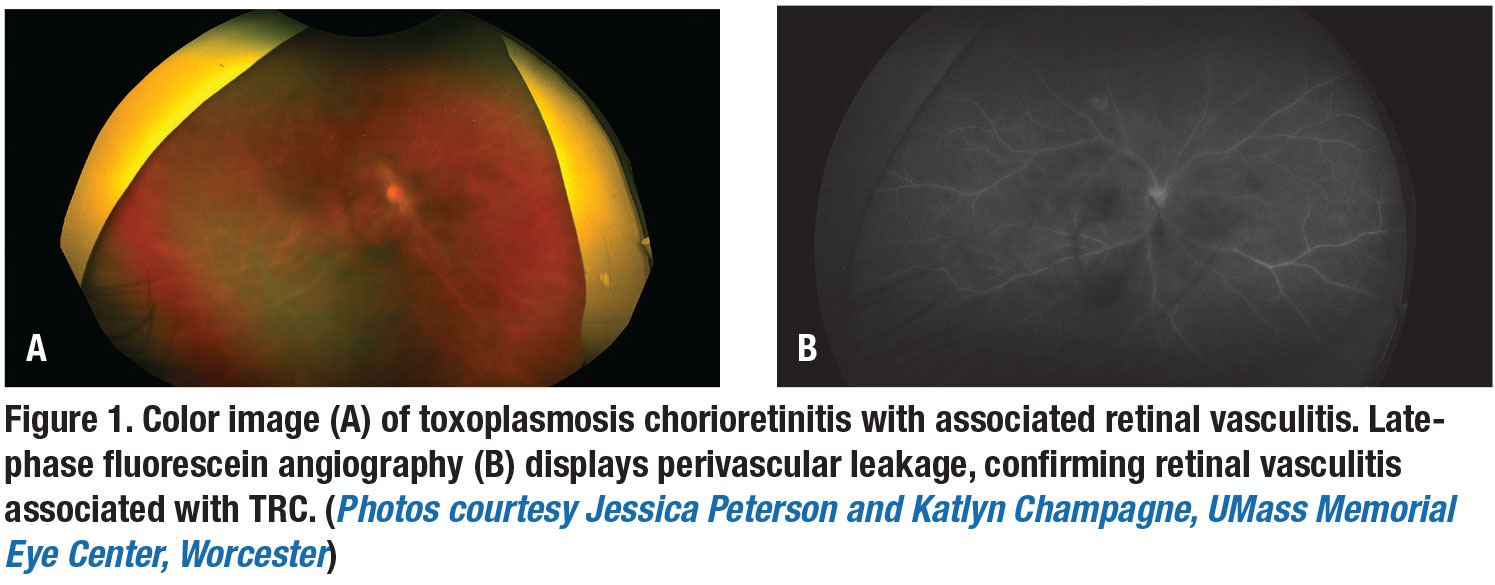
Vision loss can be due to a macular chorioretinal scar, optic-nerve involvement or severe vitreous inflammation adjacent the chorioretinal lesions. About one-third of cases can include retinal vasculitis, usually arteriolitis (Figure 1).7 In addition to posterior involvement, patients with TRC can have secondary iridocyclitis with granulomatous or stellate keratic precipitates. Secondary inflammatory ocular hypertension can develop in 10 to 15 percent of cases.1,5
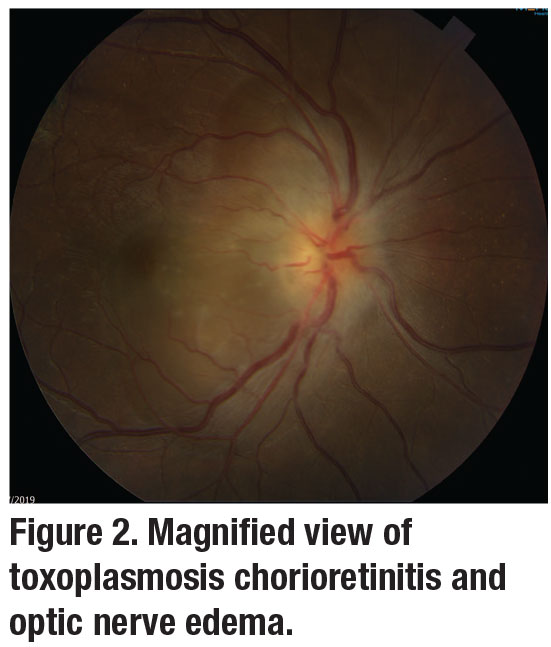
TRC may also present as neuroretinitis, punctate outer retinal toxoplasmosis, occlusive retinal vasculitis or infarction, papillitis, and retinal detachments. Though not common, optic-nerve involvement may produce severe visual field defects (Figure 2, page 14). In these unique cases, specialized lab testing can be very helpful.8,9
Diagnostic testing
The diagnosis of TRC is usually made by recognizing its distinctive clinical findings on funduscopy. In atypical cases, further lab testing with serum anti-toxoplasma antibodies, both IgM and IgG, may be needed.4,8 Toxoplasma serologic testing, when negative, can be useful to exclude toxoplasmosis as a cause of posterior uveitis; IgG has high prevalence of seropositivity in most communities, and IgM has a variable rate of decline after acutely acquired infection.8,10,11 In challenging cases, ocular fluid testing with protein catabolic rate (PCR) of aqueous or vitreous samples can also be done.1,2
Imaging as a tool
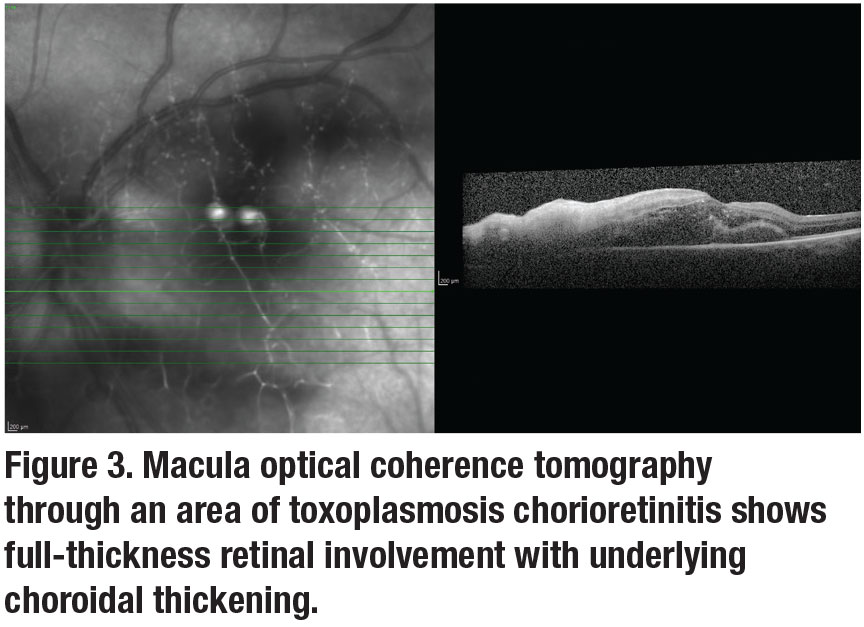
Spectral-domain optical coherence tomography imaging is important in the diagnosis and management of TRC, with changes observed based on the disease stage.9,11,12 Neurosensory retina thickening and disruption can be seen acutely. The epiretinal membrane may appear overactive or chronically scarred lesions (Figure 3).1 Collectively, fundus photography, OCT and fluorescein angiography help to document inflammation and follow complications such as macular edema, vascular occlusions and choroidal neovascularization.12
 |
Treatment options
 |
The role of systemic antibiotics for ocular toxoplasmosis has been debated extensively. Ultimately, each patient’s safety profile and location of the TRC lesion should drive the choice. TRC is self-limiting in most immunocompetent patients with a course of four to eight weeks; the risk of potential toxicity from antiparasitic medications may outweigh the benefit.7,11 Patients with small extramacular lesions that aren’t vision-threatening don’t require treatment.
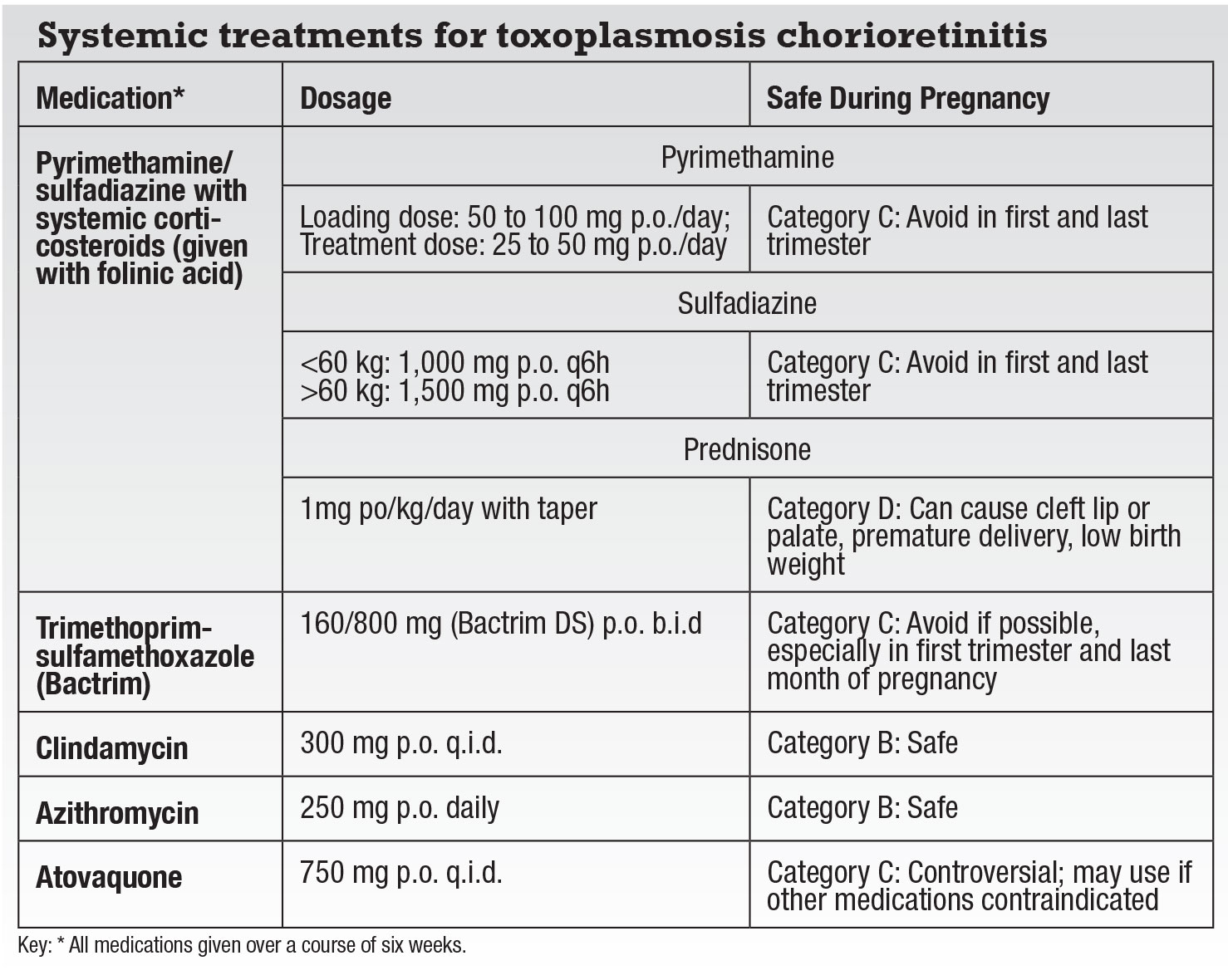
However, patients who are pregnant or immunocompromised, or have vision-threatening lesions need immediate treatment (Table). There’s also some thought that treating even extramacular lesions may reduce the risk of secondary retinal detachment.
The classic “triple-drug therapy” has long been the combination of oral pyrimethamine and sulfadiazine with systemic corticosteroids. Unfortunately, pyrimethamine can produce bone-marrow suppression in approximately 25 percent of patients as well as thrombocytopenia, rashes and fever.7 Folinic acid can limit these suppressive effects, but weekly monitoring of leukocyte and platelet counts are essential.
 |
Trimethoprim-sulfamethoxazole (Bactrim) has an overall more favorable safety profile than pyrimethamine and has been shown to be as effective in treating TRC.13 However, skin rashes occur in more than 10 percent of patients, as do rare severe hypersensitivity reactions such as Stevens-Johnson syndrome.1,7,10,11,14 Level I evidence has also shown that intermittent Bactrim (one tablet every three days) can be used as long-term prophylaxis against TRC recurrence.
The pyrimethamine/sulfadiazine and trimethoprim-sulfamethoxazole combinations are contraindicated in patients with
sulfa-drug allergies. Alternative systemic treatments with equally successful outcomes are clindamycin, azithromycin and atovaquone.7 Most of the drugs target the tachyzoites primarily, but don’t eradicate the encysted forms. Atovaquone is effective in killing both the tachyzoites and the cyst forms of toxoplasmosis, but requires close liver-function monitoring.15
 |
 |
Role of systemic corticosteroids
Systemic corticosteroids (1 mg/kg) should be started 48 hours after initiating TRC antimicrobial therapy. They’re especially helpful in recovery in patients with significant vitritis, associated optic edema and retinal vasculitis, and can help reduce inflammation surrounding the chorioretinal lesion. Corticosteroids are typically tapered slowly over four to six weeks and stopped before the antimicrobial therapy.
Intravitreal therapy
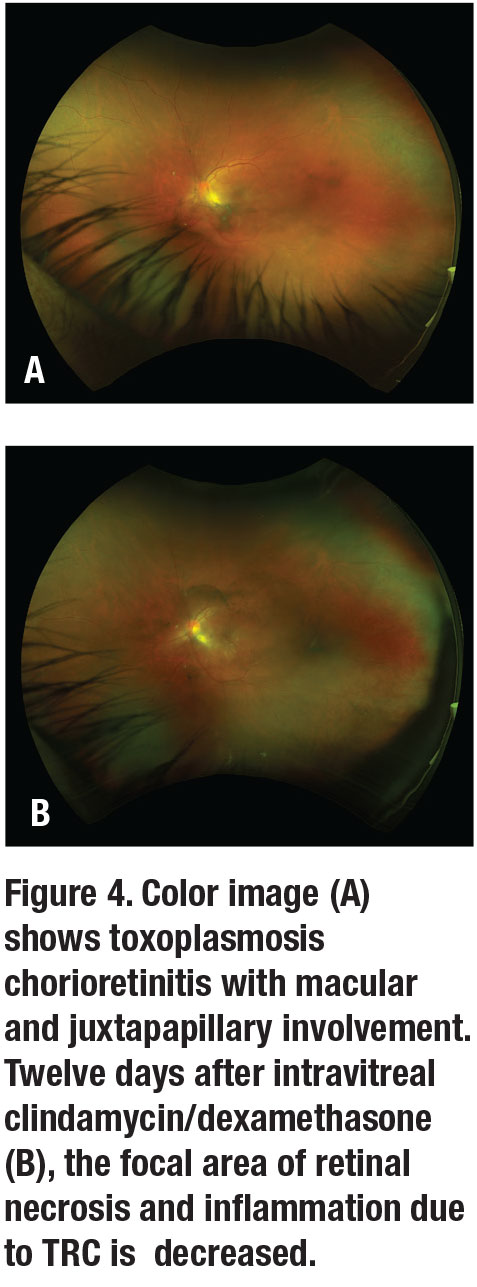
In patients with contraindications or who don’t respond to systemic therapy, a combination of intravitreal clindamycin (1 mg/0.1 mL) and dexamethasone (0.4 mg/0.1 mL) is a safe option, especially in cases with macular or juxtapapillary involvement (Figure 4). A few clinical trials have reported no difference in visual acuity, lesion size, resolution of inflammation, or recurrence between those treated with intravitreal clindamycin/dexamethasone or classic therapy of pyrimethamine, sulfadiazine and oral prednisone. Intravitreal clindamycin/dexamethasone often requires more than one dose every one to two weeks.7,16 Adding systemic therapy to pyrimethamine, sulfadiazine and oral prednisone with intravitreal clindamycin/dexamethasone hasn’t shown any additional benefit.17 Hence, intravitreal clindamycin/dexamethasone is a reasonable first-line treatment.
Bottom line
The choice of treatment for TRC depends on the patient’s immune status, drug contraindications, disease location and severity, and potential drug toxicities. RS
REFERENCES
1. Butler NJ, Furtado JM, Winthrop KL, Smith JR. Ocular toxoplasmosis II: clinical features, pathology and management. Clin Exp Ophthalmol. 2013;41:95-108.
2. Barb SM, Patel AV, Young LH. Toxoplasmic retinitis. Int Ophthalmol Clin. 2015;55:137-145.
3. Maenz M, Schlüter D, Liesenfeld O, Schares G, U Gross U, Pleyer U. Ocular toxoplasmosis past, present and new aspects of an old disease. Prog Retinal Eye Res. 2014;39:77-106.
4. Petersen E, Kijlstra A, Stanford M. Epidemiology of ocular toxoplasmosis. Ocular Immunol Inflamm. 2012;20:68-75.
5. Bosch-Driessen LE, Berendschot TT, Ongkosuwito JW, Rothova A. Ocular toxoplasmosis: clinical features and prognosis of 154 patients. Ophthalmology. 2002;109:869-878.
6. Dodds EM, Holland GN, Stanford MR, et al. Intraocular inflammation associated with ocular toxoplasmosis: relationships at initial examination. Am J Ophthalmol. 2008;146:856-865.
7. Harrrel M, Carvounis PE. Current treatment of toxoplasma retinochoroiditis: an evidence-based review. J Ophthalmol. Published online August 13, 2014.
8. Garweg JG, de Groot-Mijnes JDF, Montoya JG. Diagnostic approach to ocular toxoplasmosis. Ocular Immuno Inflamm. 2011;19:255-261.
9. Burke S, Fine H, Albini T. Toxoplasmosis retinitis masquerading as acute retinal necrosis. Ophthalmic Surg Lasers Imaging Retina. 2016;47:895-899.
10. Tabbara KF. Ocular toxoplasmosis: Toxoplasmic retinochoroiditis. Int Ophthalmol Clin. 1995;35:15-29.
11. Ozgonul C, Besirli CG. Recent developments in the diagnosis and treatment of ocular toxoplasmosis. Ophthalmic Res. 2017;57:1-12.
12. Brandão-de-Resende B, Balasundaram MB, Narain S, Mahendradas P, Vasconcelos-Santos DV. Multimodal Imaging in Ocular Toxoplasmosis. Ocular Immunol Inflamm. 2020;11:1-9.
13. Soheilian M, Sadoughi MM, Ghajarnla M, et al. Prospective randomized trial of trimethoprim/sulfamethoxazole versus pyrimethamine and sulfadiazine in the treatment of ocular toxoplasmosis. Ophthalmology. 2005;112:1876-1882.
14. Kim SJ, Scott IU, Brown GC, et al. Interventions for toxoplasma retinochoroiditis: a report by the American Academy of Ophthalmology. Ophthalmology. 2013;120:371-378.
15. Pearson PA, Piracha AR, Sen HA, Jaffe GJ. Atovaquone for the treatment of toxoplasma retinochoroiditis in immunocompetent patients. Ophthalmology. 1999; 106:148-53.
16. Sobrin L, Kump LI, Foster CS. Intravitreal clindamycin for toxoplasmic retinochoroiditis. Retina. 2007;27:952-957.
17. Bori A, Mahrous A, Al-Aswad MA, et al. Intravitreal clindamycin and dexamethasone combined with systemic oral antitoxoplasma therapy versus intravitreal therapy alone in the management of toxoplasma retinochoroiditis: A retrospective study. J Ophthalmol. Published online February 12, 2018.




