A 26-year-old Caucasian woman presented to the emergency department with the complaint of vision loss in the left eye for the past three hours. She had a history of typical migraine and had experienced “the most severe episode yet” on the day of presentation.
To help alleviate the headache she had decided to take a nap. Upon awakening, the headache had improved, but she noticed a focal area of vision loss in the superior visual field of her left eye. Her central vision was preserved. She denied flashes, floaters, pain or photophobia.
Medical and ocular history
Her ocular history was unremarkable. Her medical history was significant for migraines starting when she was a teenager, which had not been problematic for many years. She also had anxiety that was treated with Lexapro (escitalopram, Camber Pharmaceuticals). She had stopped the Lexapro a month earlier and noted an increased frequency of migraines that began to occur weekly. She was on the Nexplanon implant (etonogestrel, Merck) for birth control.
Her medical history was also significant for syncope. A previous workup had revealed a “heart murmur with a prolapse.” She denied prior episodes of blood clots, miscarriage or familial blood dyscrasias. She denied recent confusion, personality changes, focal neurologic changes or hear- ing loss. She also denied a history of oral or genital ulcers. She was a non-smoker and used alcohol and marijuana occasionally. She denied cocaine or other illicit drug use.
Retinal whitening on exam
Visual acuity was 20/20 in each eye. Intraocular pressures were normal. Pupils were equal, round and reactive with no relative afferent pupillary defect. Confrontational visual field testing revealed a superotemporal defect in her left eye. Extraocular motility was full and color plates were full in each eye. The slit lamp examination was within normal limits in both eyes. Dilated fundus exam in the left eye was notable for retinal whitening in the inferior macula extending along the inferotemporal arcade. No intra-arterial emboli or plaques were seen.
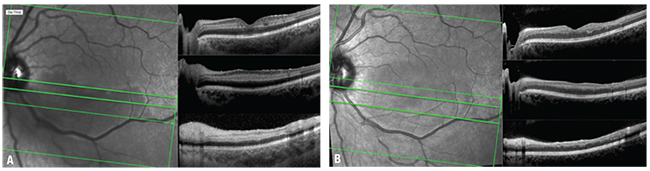 |
| Figure 1. Spectral domain optical coherence tomography at presentation shows hyper-reflective bands abutting the inner nuclear layer (A) and mild thinning and atrophy of middle retinal layers one month later (B). |
OCT finds lesions, edema
Neurology conducted a workup in the ED to evaluate for a source of embolism and stroke. This included CT of the head and neck with angiography, brain MRI and echocardiography, all of which were normal. A broad laboratory workup was unrevealing, with normal/negative findings for partial thromboplastin time, antinuclear antibody, syphilis serology, quantiferon gold, proteinase 3 antibody, myelperoxidase antibody, homocysteine, antiphospholipid antibody, protein C/S activity, antithrombin activity, prothrombin activity, cryoglobulins, serum protein electrophoresis, complete blood count, comprehensive metabolic panel and urinalysis.
Fluorescein angiography revealed no focal vasculitis or ischemic changes. The vessels in the left eye had a normal transit time and filled evenly. Fundus photography of the left eye revealed whitening along the inferior arcade extending up to but sparing the fovea. Optical coherence tomography of the left macula revealed hyper-reflective band-like lesions at the inner nuclear-layer level in addition to inner retinal edema along the inferior arcade consistent with loss of inner retinal circulation (Figure 1A).
 |
 |
Diagnosis and management
The patient was diagnosed with branch retinal artery occlusion with the associated finding of paracentral acute middle maculopathy (PAMM) extending to the parafoveal region. The close temporal proximity of the vision loss to the patient’s “most severe migraine headache to date” was suspicious for a possible connection between the two processes.
The goal of management was to prevent future similar episodes, which prompted the extensive laboratory workup. We counseled her that her visual field loss may improve, but that it may also have an irreversible component. We also suggested she avoid estrogen-containing hormonal contraceptives and smoking, given the demonstrated propensity for possible thrombotic events. She decided to discontinue her progesterone-based contraceptive on her own.
After discussion with her neurologist, she was also started on topiramate as prophylaxis against future migraine episodes.
We reexamined the patient one month later, and the first follow-up OCT showed atrophic thinning of the affected retina (Figure 1B).
Findings in the middle retinal layer
In 2014 Suqin Yu, MD, and colleagues described abnormal bands of hyper-reflectivity on spectral domain OCT as a marker of retinal ischemia, with hyper-reflectivity of the superficial capillary plexus corresponding to cotton wool spots, and hyper-reflective bands in the middle retinal layer corresponding to deeper foci of retinal whitening.1 In 2015 David Sarraf, MD, and colleagues described similar hyper-reflective bands in the middle retinal layers on OCT that lacked angiographic correlation, which have since been termed PAMM.2,3
PAMM is a finding on SD-OCT characterized by hyper-reflective band-like lesions at the inner nuclear layer in patients with an acute negative scotoma. The retinal capillary network is composed of the superficial capillary plexus, located at the ganglion cell layer and the intermediate and deep capillary plexuses at the level of the inner nuclear layer.
 |
| Figure 2. Fundus photography at presentation (A) shows retinal edema along the inferior arcade with retinal whitening extending up to the parafoveal region. At one month follow-up (B), the edema resolved and vascular attenuation of the arteries of the inferior arcade increased. |
Pathophysiologically, PAMM is thought to result from localized retinal capillary ischemia at the level of the intermediate or deep plexuses, resulting in localized edema surrounding the inner nuclear layer.4,5 OCT angiography has produced new insights into both retinal vascular anatomy and clinical correlations when select structures are affected, such as in PAMM.6 In this condition, deep capillary plexus abnormalities appear on OCTA.
Analogous to cotton wool spot
The presence of PAMM serves as a sign of an underlying vascular disease. It is not considered a diagnosis, analogous to a cotton wool spot. It has been reported in patients with BRAO, central retinal vein or artery occlusion, diabetic retinopathy, hypertensive retinopathy, sickle cell retinopathy, Purtscher’s retinopathy, retinal vasculitis, carotid embolism, migraines, medication toxicity, hypovolemia, orbital compression injury and viral illness.4,8
The designation of PAMM is made on the basis of a combination of a clinical history of acute scotoma with the described SD-OCT findings. PAMM lesions may be seen on fundus examination as whitish parafoveal deep retinal lesions that are smoother and more grayish than cotton wool spots. Patients with vascular risk factors typically present in their 50s or 60s. In younger patients, however, PAMM is often idiopathic. Although there is no specific treatment for PAMM, it is important to take a thorough history and appropriately work-up patients to rule out systemic contributions.4
 |
Follow-up and bottom line
One month after her initial presentation, our patient still noticed the “blind spot” present in her initial visit, although she characterized it as lighter and less opaque. Typically, a partial scotoma is the usual outcome in these patients, and they should be counseled accordingly.8,11
Our patient highlights the importance of recognizing PAMM in cases of unexplained vision loss. In particular, pay attention to findings of hyper-reflectivity of the middle retinal layers on OCT. Although no definitive evidence links migraine with BRAO or PAMM, prior case reports have demonstrated a similar clinical experience.8,12 Future studies utilizing OCTA and other imaging modalities may help us to better understand the responsible vascular alterations, particularly if captured in the acute setting. RS
REFERENCES
1. Yu S, Wang F, Pang CE, Yannuzzi LA, Freund KB. Multimodal imaging findings in retinal deep capillary ischemia. Retina. 2014;34:636-646.
2. Sarraf D, Rahimy E, Fawzi AA, et al. Paracentral acute middle maculopathy: a new variant of acute macular neuroretinopathy associated with retinal capillary ischemia. JAMA Ophthalmol. 2013;131:1275-1287.
3. Rahimy E, Kuehlewein L, Sadda SR, Sarraf D. Paracentral acute middle maculopathy: what we knew then and what we know now. Retina. 2015;35:1921-1930.
4. Pecen PE, Smith AG, Ehlers JP. Optical coherence tomography angiography of acute macular neuroretinopathy and paracentral acute middle maculopathy. JAMA Ophthalmol. 2015;133:1478-1480.
5. Dansingani KK, Inoue M, Engelbert M, Freund KB. Optical coherence tomographic angiography shows reduced deep capillary flow in paracentral acute middle maculopathy. Eye (Lond). 2015;29:1620-1624.
6. Khan MA, Rahimy E, Shahlaee A, Hsu J, Ho AC. En face optical coherence tomography imaging of deep capillary plexus abnormalities in paracentral acute middle maculopathy. Ophthalmic Surg Lasers Imaging Retina. 2015;46:972-975.
7. Dansingani KK, Freund KB. Paracentral acute middle maculopathy and acute macular neuroretinopathy: related and distinct entities. Am J Ophthalmol. 2015;160:1-3 e2.
8. Chen X, Rahimy E, Sergott RC, et al. Spectrum of retinal vascular diseases associated with paracentral acute middle maculopathy. Am J Ophthalmol. 2015;160:26-34.
9. Casalino G, Arrigo A, Romano F, Munk MR, Bandello F, Parodi MB. Acute macular neuroretinopathy: pathogenetic insights from optical coherence tomography angiography. Br J Ophthalmol. 2018 May 28 [epub ahead of print].
10. Thanos A, Faia LJ, Yonekawa Y, Randhawa S. Optical coherence tomography in acute macular neuroretinopathy. JAMA Ophthalmol. 2016;134:1310-1314.
11. Chen Y, Hu Y. The optical imaging of idiopathic paracentral acute middle maculopathy in a Chinese young man and review of the literature. Photodiagnosis Photodyn Ther. 2017;19:383-387.
12. Beversdorf D, Stommel E, Allen CN, Stevens R, Lessell S. Recurrent branch retinal infarcts in association with migraine. Headache. 1997;37:396-369.



