ABOUT THE AUTHOR | ||
 | Dr. Khurana is a partner with Northern California Retina Vitreous Associates and a clinical assistant professor of ophthalmology at the University of California, San Francisco. | |
However, these implants can occasionally migrate into the anterior chamber, causing vision-threatening complications that involve permanent corneal decompensation.5-7 The initial reports were in aphakic eyes, and authors speculated that aphakia is the main risk factor for anterior chamber complications.6
Risk Factors for Migration
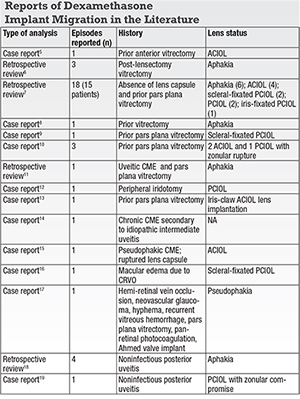
The largest series of DEX implant anterior chamber migration reviewed the risk factors, clinical outcomes and management strategies.7 The authors described 15 patients with 18 episodes of anterior chamber migration of the DEX implant. All eyes had been treated for cystoid macular edema from various causes including central retinal vein occlusion (CRVO), branch retinal vein occlusion (BRVO), non-infectious uveitis and DME. All cases had a history of vitrectomy and either an absent or defective lens capsule. All 15 patients had prior pars plana vitrectomy (PPV) and 14 (93 percent) had no lens capsule. Six eyes were aphakic, four had an anterior-chamber intraocular lens (ACIOL) (Figure), two had a scleral-fixated IOL, two had a posterior-chamber IOL (PCIOL) and one had an iris-fixated PCIOL. Five patients had prior uncomplicated dexamethasone implant injections with the same lens and capsule status.7The average number of days from injection to migration was 13 (range, five to 44 days). Corneal edema was the most significant vision-threatening complication, which presented in all but two cases. In all the patients presenting with corneal edema, the time from injection to migration was 19 days or less.7
In one case without corneal edema, the implant was lodged between the iris and the lens capsule, so it was not in proximity to the corneal endothelium. In the other case without corneal edema, the implant had migrated anteriorly 44 days after injection, presumably after significant degradation.
When the implant migrated into the anterior chamber, one patient was observed, four were positioned supinely, one underwent YAG fragmentation and 11 underwent surgical removal involving either forceps, aspiration of implant fragments or repositioning into the posterior chamber. The implant can be friable and so can be a challenge to remove in one piece. It was aspirated with a vitreous cutter.7
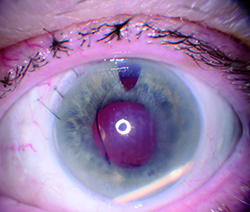 |
| Figure. In this eye with an anterior chamber intraocular lens, the dexamethasone implant has migrated into the anterior chamber and corneal edema is present. (Image courtesy of David J. Parks, MD) |
In this report of 15 patients with anterior chamber migration of the DEX implant, the high rate of permanent corneal edema development is most concerning. Anterior migration occurred in eyes that were aphakic and pseudophakic with the lens capsule either absent or compromised. Surgery was successful in removing the implant, but had limited success in reversing the corneal edema. Treating retina specialists should be aware of this potential adverse event, and that the possible risk factors include not just aphakia, but any degree of compromised or absent capsule, even with an IOL and a history of vitrectomy surgery.
Anterior chamber migration of the DEX implant was first reported in a vitrectomized eye with an ACIOL and then in aphakic vitrectomized eyes.5,6 Subsequently, cases involving PCIOLs were also published.8-10 In contrast, the Phase III clinical studies involving the DEX implant for RVO and DME reported no cases of anterior chamber migration of the steroid implant in 2,335 patients.1,3 However, the exclusion criteria for entry into the study included aphakia or ACIOL.
Clinical Pearls |
| • Combination of vitrectomy and lens capsular/zonular defect puts patients at risk. • IOL will not preclude implant migration. • Migration puts patients at high risk for permanent corneal edema and decompensation. • Urgent removal of the migrated implant with corneal edema is recommended. |
Anterior chamber migration occurs in eyes with aphakia and pseudophakia with anterior chamber, iris-fixated, scleral-fixated and intracapsular posterior chamber IOLs. The two common risk factors in the previously referenced series were a defective or absent posterior capsule and a prior vitrectomy.7 Nevertheless, the presence of both of these risk factors does not necessarily guarantee that implant migration will occur.
Five patients in the previously referenced series had prior uncomplicated DEX implant injections without anterior chamber migration and had the same lens and capsular status when migration occurred later.7 Further, one study speculated that zonular dehiscence and large peripheral iridotomy may play a role when the implant migrates into the anterior chamber.19 We should monitor patients with these conditions closely for migration of the implant.
To prevent migration of the DEX implant in patients with these risk factors, some investigators have proposed a technique of scleral fixation of the DEX implant in the vitreous cavity with a 10-0 nonabsorbable polypropylene suture.20
Complications of Migration
Corneal edema is the most serious complication of DEX implant migration in the anterior chamber. In the series noted earlier, 16 cases (89 percent) of corneal edema were observed at presentation among the 18 episodes of DEX implant migration.7 In 10 patients (71 percent), the corneal edema did not spontaneously resolve despite implant removal, and six (43 percent) were referred for keratoplasty.7The mechanism of corneal edema could be endothelial decompensation either due to chemical toxicity from any component of the DEX implant (dexamethasone, lactic acid or glycolic acid) or mechanical trauma from a rigid object. Specular microscopy has demonstrated corneal endothelial cell loss when the DEX implant migrates in the anterior chamber.10
High doses of dexamethasone are cytotoxic to corneal endothelial cells, inducing both apoptosis and necrosis.21 Corneal edema occurs with early migration (within less than three weeks).7
Two previously reported cases in which the implant migrated at five weeks and three months after injection did not develop corneal edema.6,12 With late migration (occurring after three weeks), the implant may have degraded enough to cause minimal corneal toxicity from the dexamethasone, but further study is required.
Anterior chamber migration is not unique to the DEX implant. It was first reported after intravitreal injection of triamcinolone acetonide, resulting in a pseudohypopyon with the steroid crystals in the anterior chamber.22,23 Anterior chamber migration is also possible with another intravitreal device involving the fluocinolone acetonide implant (Iluvien, Alimera Sciences).
The FDA approved Iluvien in 2014 for treatment of DME in patients previously treated with corticosteroids who did not have a rise in intraocular pressure. It contains 0.19 mg of fluocinolone acetonide and is injected into the vitreous cavity. Because it is a free-floating implant (and non-tethered like the DEX implant), Iluvien can migrate into the anterior chamber in vitrectomized eyes. Cases of anterior chamber migration have been reported, but none have been published.
The package insert for Iluvien carries a warning that notes that patients in whom the posterior capsule is either absent or torn are at risk of implant migration into the anterior chamber. The incidence of corneal issues with the fluocinolone acetonide implant that migrates anteriorly is not known.
Retina specialists should be aware of this potential complication with the fluocinolone acetonide implants and exercise caution in the setting of anterior chamber migration and corneal edema.
Management Options
A few management options exist in the setting of anterior chamber migration of the DEX implant. Observation may be considered in the absence of corneal edema. Supine positioning of the patient and pupil dilation in aphakic eyes may allow the implant to fall back posteriorly.14Pilocarpine may reduce pupil size either primarily or following repositioning to minimize the chance of the implant moving anteriorly, but recurrent migration may still occur.6 A slit-lamp procedure using a needle to reposition the implant posteriorly is an option as well, but the implant may return.13 Typically, no corneal edema is present with late migration (after three weeks), so in these cases observation could be employed.
If the implant does migrate into the anterior chamber and corneal edema ensues, it should be removed as soon as possible to prevent corneal decompensation. Early removal of the DEX implant has been known to reduce the likelihood of permanent corneal endothelial damage.7 This suggests that removing the implant earlier is better for the corneal endothelium and preventing chronic corneal edema.
With regards to the surgical technique for removing the DEX implant, an anterior segment approach using viscoelastic to coax the implant toward a limbal incision can be used if the implant is not friable. Once it moves to the incision, the implant can be removed by gently grasping it with a forceps.
An elegant surgical technique involving standard vitreoretinal instrumentation, viscoelastic, a modified Sheets glide and angled forceps to avoid fragmentation of the implant and limit iatrogenic morbidity has also been described.26 A helpful surgical pearl involves using an angled McPherson forceps to grasp the implant parallel to its long axis and avoid dissolution of the implant into multiple particulate fragments.26
A friable implant can be difficult to grasp with forceps or other surgical instruments, and it could disintegrate into numerous fragments with minimal manipulation. In these situations, aspiration with a vitreous cutter or similar instrument may be necessary to remove the implant. Be prepared for these scenarios and anticipate the possible need for vitreoretinal instrumentation when attempting to remove the implant.7
The package insert for the dexamethasone implant was modified in September 2012 to reflect that contraindications include aphakia and an ACIOL with rupture of the posterior capsule. However, the combination of compromised zonular or posterior capsular integrity and a history of vitrectomy makes anterior migration of the DEX implant more likely.
It is important to emphasize that the presence of an IOL alone will not prevent migration in the absence of an intact posterior lens capsule. When migration occurs, the patient is at risk for corneal edema and decompensation. If corneal edema does occur, I recommend urgent removal of the DEX implant. RS
Disclosures: Dr. Khurana disclosed that he is a consultant and investigator for Allergan; a consultant and speaker for Genentech; and a consultant, speaker and investigator for Regeneron.
References
1. Haller JA, Bandello F, Belfort R Jr., et al. Randomized, sham-controlled trial of dexamethasone intravitreal implant in patients with macular edema due to retinal vein occlusion. Ophthalmology. 2010;117:1134-1146 e1133.2. Lowder C, Belfort R Jr., Lightman S, et al. Dexamethasone intravitreal implant for noninfectious intermediate or posterior uveitis. Arch Ophthalmol. 2011;129:545-553.
3. Boyer DS, Yoon YH, Belfort R Jr., et al. Three-year, randomized, sham-controlled trial of dexamethasone intravitreal implant in patients with diabetic macular edema. Ophthalmology. 2014;121:1904-1914.
4. Boyer DS, Faber D, Gupta S, et al. Dexamethasone intravitreal implant for treatment of diabetic macular edema in vitrectomized patients. Retina. 2011;31:915-923.
5. Pardo-Lopez D, Frances-Munoz E, Gallego-Pinazo R, Diaz-Llopis M. Anterior chamber migration of dexamethasone intravitreal implant (Ozurdex®). Graefes Arch Clin Exp Ophthalmol. 2011.
6. Bansal R, Bansal P, Kulkarni P, Gupta V, Sharma A, Gupta A. Wandering Ozurdex® implant. J Ophthalmic Inflamm Infect. 2012;2:1-5.
7. Khurana RN, Appa SN, McCannel CA, et al. Dexamethasone implant anterior chamber migration: risk factors, complications, and management strategies. Ophthalmology. 2014;121:67-71.
8. Cronin KM, Govind K, Kurup SK. Late migration of dexamethasone implant into anterior chamber. Arch Ophthalmol. 2012;130:711.
9. Voykov B, Bartz-Schmidt KU. Dislocation of dexamethasone intravitreous implant. Arch Ophthalmol. 2012;130:706.
10. Malcles A, Janin-Manificat H, Yhuel Y, et al. [Anterior chamber migration of intravitreal dexamethasone implant (Ozurdex®) in pseudophakic eyes: Report of three cases.]. J Fr Ophtalmol. 2013;36:362-367.
11. Adan A, Pelegrin L, Rey A, et al. Dexamethasone intravitreal implant for treatment of uveitic persistent cystoid macular edema in vitrectomized patients. Retina. 2013;33:1435-1440.
12. Eadie JA LR, Capone, Jr A. Migration of Ozurdex implant into the anterior chamber. Retinal Cases and Brief Reports. 2012;6:269-270.
13. Vela JI, Crespi J, Andreu D. Repositioning of dexamethasone intravitreal implant (Ozurdex®) migrated into the anterior chamber. Int Ophthalmol. 2012;32:583-584.
14. Kishore SA, Schaal S. Management of anterior chamber dislocation of dexamethasone implant. Ocul Immunol Inflamm. 2013;21:70-71.
15. Marin-Lambies C, Gallego-Pinazo R, Garcia-Delpech S, Diaz-Llopis M. [Ozurdex® and aphakia: a combination to avoid.] Arch Soc Esp Oftalmol. 2012;87:191-192.
16. Laplace O, Rodallec T, Akesbi J, Sandali O. [Anterior chamber migration of a dexamethasone implant in a pseudophakic patient with a scleral-fixated posterior chamber intraocular lens]. J Fr Ophtalmol. 2013;36:e59-61.
17. Collet B. Management of Ozurdex in the anterior chamber. JAMA Ophthalmol. 2013;131:1651-1652.
18. Bratton ML, He YG, Weakley DR. Dexamethasone intravitreal implant (Ozurdex) for the treatment of pediatric uveitis. JAAPOS. 2014;18:110-113.
19. Kocak N, Ozturk T, Karahan E, Kaynak S. Anterior migration of dexamethasone implant in a pseudophakic patient with intact posterior capsule. Indian J Ophthalmol. 2014;62:1086-1088.
20. Mateo C, Alkabes M, Bures-Jelstrup A. Scleral fixation of dexamethasone intravitreal implant (Ozurdex®) in a case of angle-supported lens implantation. International ophthalmology. 2014;34:661-665.
21. Chen WL, Lin CT, Yao CC, et al. In-vitro effects of dexamethasone on cellular proliferation, apoptosis, and Na+-K+-ATPase activity of bovine corneal endothelial cells. Ocul Immunol Inflamm. 2006;14:215-223.
22. Ruiz-Moreno JM, Montero JA, Artola A, Barile S. Anterior chamber transit of triamcinolone after intravitreal injection. Arch Ophthalmol. 2005;123):129-130.
23. Moshfeghi AA, Scott IU, Flynn HW, Jr., Puliafito CA. Pseudohypopyon after intravitreal triamcinolone acetonide injection for cystoid macular edema. Am J Ophthalmol. 2004;138:489-492.
24. Akduman L, Cetin EN, Levy J, Becker MD, Mackensen F, Lim LL. Spontaneous dissociation and dislocation of Retisert pellet. Ocul Immunol Inflamm. 2013;21:87-89.
25. Chang PY, Kresch Z, Samson CM, Gentile RC. Spontaneous dissociation of fluocinolone acetonide sustained release implant (Retisert) with dislocation into the anterior chamber. Ocul Immunol Inflamm. 2014:11:1-4.
26. Stelton CR TJ, Peterson LT, Khurana RN, Yeh S. Surgical management of anterior chamber migration of a dexamethasone intravitreal implant. Ophthalm Surg Lasers Imaging Retina. 2015. Submitted for publication.



