Focus on Digital Medicine in Retina
Take-home points
|
 |
|
Bios Dr. Salman is a chief ophthalmology resident at Mayo Clinic in Rochester, Minnesota. Dr. Barkmeier is an associate professor of ophthalmology at Mayo Clinic in Rochester. DISCLOSURES: Dr. Salman and Dr. Barkmeier have no conflicting relationships to disclose. |
Telemedicine has demonstrated significant potential to reduce barriers and improve adherence for diabetic retinopathy screening of asymptomatic patients in the primary-care setting.1–3 However, one of the main limiting factors of telemedicine for DR screening is access to accurate and timely image interpretation.4 This is where artificial intelligence can play a significant role.
AI-based image analysis has shown the potential to deliver accurate, real-time fundus photography grading, allowing health systems to reduce the number of routine screening eye care referrals.1,2,5–7
The scope of the problem has been well documented: More than 100 million people have diabetes8 and an estimated 860,000 are functionally blind from DR. An additional 2.9 million suffer from moderate to severe visual impairment.9 Although systemic management of diabetes mellitus has improved significantly over the past few decades, global prevalence continues to rise and with it the burden of DR. It remains the leading cause of new legal blindness among Americans ages 20 to 74 years.1,2,9–11
Here, we report on the advances made in using telemedicine for screening for DR and future directions.
Poor adherence to guidelines
Regular monitoring and early detection is the key to preventing DR-related visual impairment. American Academy of Ophthalmology guidelines recommend screening for DR at the time of diagnosis for patients with T2DM and at least annually starting five years after diagnosis of T1DM. Unfortunately, only about 60 percent of patients with DM receive eye exams at least yearly.12 This discrepancy is multifactorial: the financial burden of follow-up visits, inconvenience of traveling to a doctor’s office, timing and duration of visits, and limitations in patient education.1,3,13 An additional complicating factor is that many patients remain asymptomatic even when they have advanced, vision-threatening DR.14
In the United States, telemedicine systems have been implemented for screening and/or managing DR, as well as in emergency medicine teleophthalmology, retinopathy of prematurity screening, and management of age-related macular degeneration and glaucoma—with varying levels of success.15
The Veterans Health Administration implemented a nonmydriatic teleretinal DR screening that has shown efficacy in reaching a larger population of patients while proving cost-
effective for screening in populations of more than 3,500 patients under age 80 from diverse racial ethnic groups.16
Dilation and image gradeability
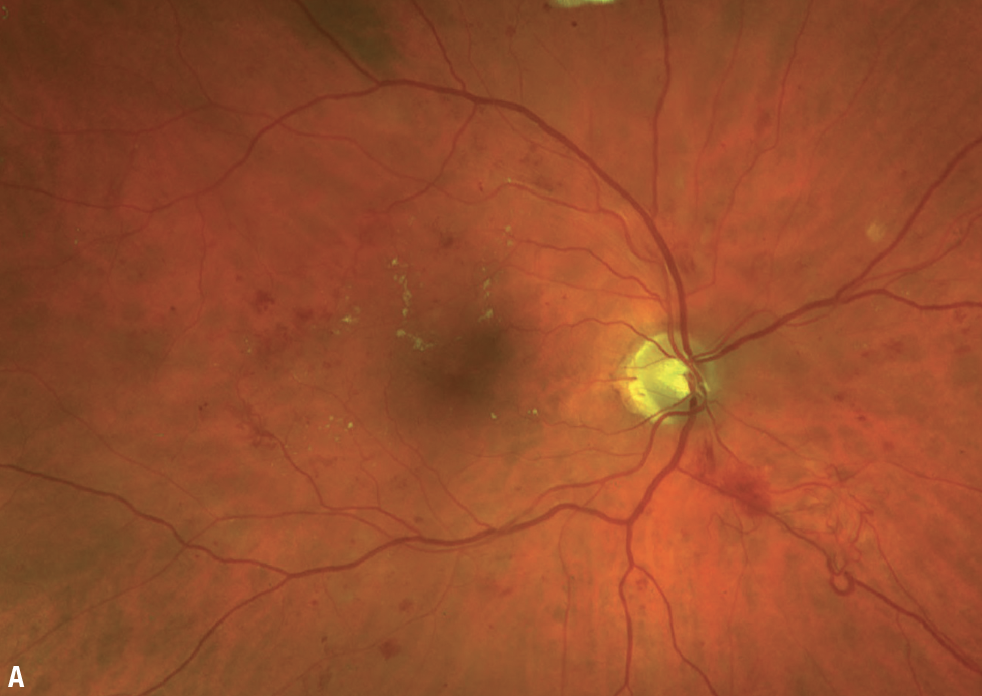 |
| Figure A. Ultrawide pseudocolor fundus photography of a 71-year-old man with newly diagnosed noninsulin-dependent diabetes mellitus reveals intra- and extramacular dot and blot hemorrhages, macular exudates, intraretinal microvascular abnormalities and neovascularization elsewhere. |
Teleophthalmology programs can use both mydriatic and nonmydriatic fundus photography. Dilation has the most significant impact on telemedicine image quality in older patients and those with known ocular media opacity. A recent study found the rate of gradable nonmydriatic images fell from 83 percent for patients ages <40 years to 50 percent for patients ages 61 to 70 (p<0.001) and 33 percent for those ages 71 to 80 (p<0.001).17
Although postdilation fundus images are typically of higher quality than nonmydriatic images, dilation adds time and cost for both patients and health-care
systems, and may be inconvenient and uncomfortable for many patients.18 Another potential concern regarding pharmacologic mydriasis in the primary-care setting is the risk of inducing acute angle closure due to pupillary block, although this has been exceedingly rare with tropicamide-only dilation.19
Screening programs in which ungradable nonmydriatic photography leads directly to a referral must balance the benefits of avoiding routine dilation with the costs of increased office visits and the risk of losing patients to follow-up.
Track record of AI algorithms
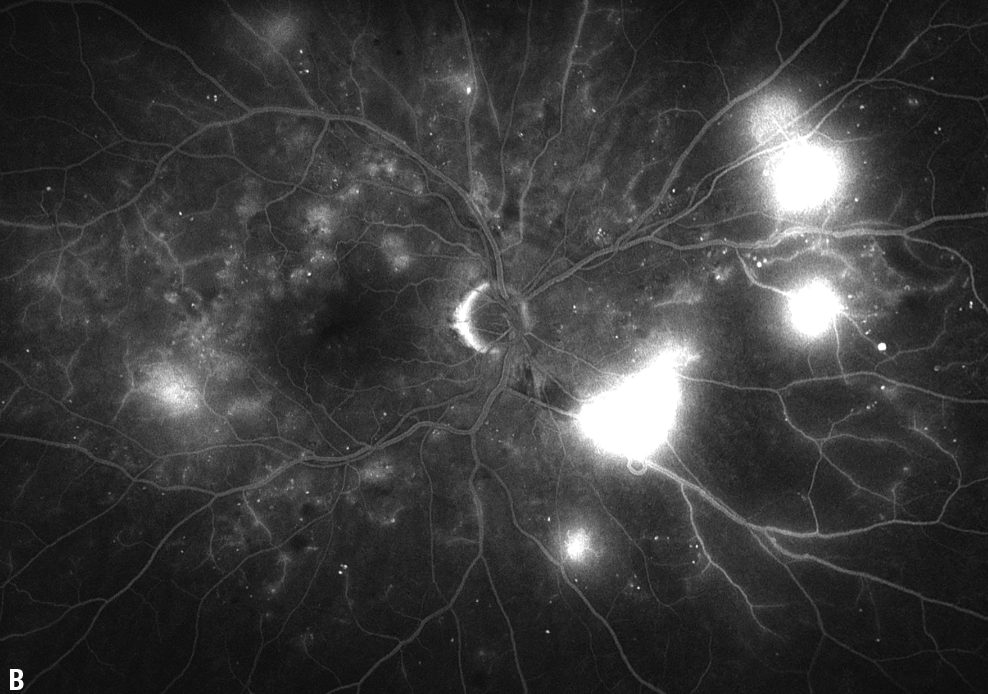 |
| Figure B. Fluorescein angiography demonstrates several areas of late leakage as well as scattered areas of small-vessel leakage and vascular nonperfusion. |
A recent, large-scale international prospective study demonstrated that an AI-based deep-learning program could offer 91.4-percent sensitivity for detecting vision-threatening DR with a specificity of 95.4 percent, which was at least as good as retina specialist grading.20
Another recent study compared the performance of seven different AI-based DR screening algorithms on real-world Veterans Affairs patient data and found widely varying sensitivities—51 to 86 percent.21 Negative predictive values (NPV) ranged from 82.7 to 93.7 percent.
Two of the algorithms had slightly higher sensitivities than the teleretinal human grader control, although the algorithms had lower specificities. One algorithm had worse performance at all levels of DR severity compared with teleretinal control and missed a quarter of advanced retinopathy cases. These findings highlight the need for rigorous real-world algorithm validation before they’re implemented in the clinic.
The VA study calculated value per encounter, defined as the cost saved avoiding unnecessary referrals to a provider, for all algorithms that performed no worse than teleretinal graders in detecting referable DR. This value was found to be $15 to $18 per screening visit for ophthalmologist human graders and $8 to $9 for optometrists.21
 |
Existing AI-based systems
Two AI-based systems approved for DR screening are available commercially: the IDx-DR autonomous diagnostic platform (Digital Diagnostics); and the EyeArt AI screening system (Eyenuk).
IDx-DR analyzes two fundus photographs of each eye using the TRC 400NW 70 nonmydriatic fundus camera (Topcon) to identify patients with more than mild DR.6 A retrospective study reported outcomes following incorporation of IDx-DR AI-based image interpretation into an established DR telemedicine screening program.17 It evaluated 1,052 consecutive adult patients who received photoscreening for DR in a primary-care setting. IDx-DR analyzed nonmydriatic fundus photos captured for each patient.
When the program couldn’t grade the nonmydriatic photos, the patients were dilated (1% tropicamide). The AI platform successfully analyzed the mydriatic fundus photos in 87.5 percent of patients who had ungradable nonmydriatic photos.17 More than 90 percent ultimately had gradable fundus photos and 14.3 percent were graded as greater-than-mild DR.
Manual over-read was performed on all images. The AI-derived results compared to manual over-read had 100-percent sensitivity, 89.2-percent specificity and 100- percent NPV for identifying more than mild nonproliferative DR.17
A prospective trial last year showed the EyeArt system also had high sensitivity (95.5 percent) and specificity (85 percent) for detecting more-than-mild and vision-threatening DR (95.1-percent sensitivity, 89-percent specificity). Nearly 90 percent of eyes didn’t require dilation for the AI algorithm to identify mild-to-moderate and vision-threatening DR.22
Potential to find referable disease
Findings from these studies demonstrate the potential of AI-based image interpretation systems to identify referral-eligible disease with a very high sensitivity and high NPV. The low false-negative rate may allow telemedicine systems to either arrange a secondary review of only positive screening images or eliminate the secondary review only of images with positive screening results, or to even entirely eliminate the secondary review if the system can accommodate prompt access to dilated comprehensive eye exams for all patients with positive or ungradable results.
 |
Introducing these systems for screening of patients with diabetes in the primary-care setting holds great potential for improving access to care. The unique characteristics and infrastructure of different systems will determine specific practices, such as how to manage positive or ungradable screenings either through secondary image review or with urgent referral of all patients with positive or ungradable screening results.
Patients with negative results may either continue getting telemedicine screening annually or some systems may recommend they get periodic comprehensive eye exams on a less-than-annual basis. Importantly, systems must be put in place to streamline referrals when indicated.
Every effort should be made to minimize the obstacles to getting patients into the clinic to avoid liability issues, including obtaining valid contact information on all and ensuring patients are fully informed on the potential risks of AI-based screening as well as the fact that they may still need an eye exam. Obstacles to wider implementation of AI-based screening programs include regulatory issues, variation in software and discrepancies in national screening programs.20
Bottom line
Incorporating AI-based image analysis into primary-care DR screening programs has the potential to improve patient access to recommended screening with a high sensitivity for detecting retinopathy warranting referral for a comprehensive eye examination. RS
REFERENCES
1. Barkmeier AJ. Toward optimal screening for diabetic retinopathy: Balancing precision and pragmatism. Mayo Clin Proc. 2021;96:282-284.
2. Vujosevic S, Aldington SJ, Silva P, et al. Screening for diabetic retinopathy: new perspectives and challenges. Lancet Diabetes Endocrinol. 2020;8:337-347.
3. Wong TY, Sun J, Kawasaki R, et al. Guidelines on diabetic eye care: The International Council of Ophthalmology recommendations for screening, follow-up, referral, and treatment based on resource settings. Ophthalmology. 2018;125:1608-1622.
4. Ting DS, Cheung GC, Wong TY. Diabetic retinopathy: global prevalence, major risk factors, screening practices and public health challenges: a review. Clin Exp Ophthalmol. 2016;44:260-277.
5. Grauslund J. Diabetic retinopathy screening in the emerging era of artificial intelligence. Diabetologia. 2022;65:1415-1423.
6. Grzybowski A, Brona P, Lim G, et al. Artificial intelligence for diabetic retinopathy screening: a review. Eye (Lond). 2020;34:451-460.
7. Gunasekeran DV, Ting DSW, Tan GSW, Wong TY. Artificial intelligence for diabetic retinopathy screening, prediction and management. Curr Opin Ophthalmol. 2020;31:357-365.
8. Teo ZL, Tham YC, Yu M, et al. Global Prevalence of Diabetic Retinopathy and Projection of Burden through 2045: Systematic Review and Meta-analysis. Ophthalmology. 2021;128:1580-1591.
9. GBD 2019 Blindness and Vision Impairment Collaborators; Vision Loss Expert Group of the Global Burden of Disease Study. Causes of blindness and vision impairment in 2020 and trends over 30 years, and prevalence of avoidable blindness in relation to VISION 2020: the Right to Sight: an analysis for the Global Burden of Disease Study. Lancet Glob Health. 2021;9:e144-e160.
10. Leasher JL, Bourne RR, Flaxman SR, et al. Global estimates on the number of people blind or visually impaired by diabetic retinopathy: A meta-analysis from 1990 to 2010. Diabetes Care. 2016;39:1643-1649.
11. Yau JW, Rogers SL, Kawasaki R, et al. Global prevalence and major risk factors of diabetic retinopathy. Diabetes Care. 2012;35:556-564.
12. Flaxel CJ, Adelman RA, Bailey ST, et al. Diabetic Retinopathy Preferred Practice Pattern. Ophthalmology. 2020;127:66-145.
13. Kashim RM, Newton P, Ojo O. Diabetic retinopathy screening: A systematic review on patients' non-attendance. Int J Environ Res Public Health. 2018;15:157.
14. Cheung N, Mitchell P, Wong TY. Diabetic retinopathy. Lancet. 2010;376:124-136.
15. Rathi S, Tsui E, Mehta N, Zahid S, Schuman JS. The current state of teleophthalmology in the United States. Ophthalmology. 2017;124:1729-1734.
16. Kirkizlar E, Serban N, Sisson JA, Swann JL, Barnes CS, Williams MD. Evaluation of telemedicine for screening of diabetic retinopathy in the Veterans Health Administration. Ophthalmology. 2013;120:2604-2610.
17. Mehra AA, Softing A, Guner MK, Hodge DO, Barkmeier AJ. Diabetic retinopathy telemedicine outcomes with artificial intelligence-based image analysis, reflex dilation, and image overread. Am J Ophthalmol. Published online August 12, 2022. doi: 10.1016/j.ajo.2022.08.008
18. Han YS, Pathipati M, Pan C, et al. Comparison of telemedicine screening of diabetic retinopathy by mydriatic smartphone-based vs nonmydriatic tabletop camera-based fundus imaging. J Vitreoretin Dis. 2021;5:199-207.
19. Pandit RJ, Taylor R. Mydriasis and glaucoma: exploding the myth. A systematic review. Diabet Med. 2000;17:693-699.
20. Ruamviboonsuk P, Tiwari R, Sayres R, et al. Real-time diabetic retinopathy screening by deep learning in a multisite national screening programme: a prospective interventional cohort study. Lancet Digit Health. 2022;4:e235-e244.
21. Lee AY, Yanagihara RT, Lee CS, et al. Multicenter, head-to-head, real-world validation study of seven automated artificial intelligence diabetic retinopathy screening systems. Diabetes Care. 2021;44:1168-1175.
22. Ipp E, Liljenquist D, Bode B, et al. Pivotal evaluation of an artificial intelligence system for autonomous detection of referrable and vision-threatening diabetic retinopathy. JAMA Netw Open. 2021;4:e2134254.



