Take-home points
|
 |
|
Bios Dr. Cakir is a research fellow at The Tony and Leona Campane Center for Excellence in Image-Guided Surgery and Advanced Imaging Research, Cole Eye Institute, Cleveland Clinic, Cleveland. Dr. Ehlers holds the Norman C. and Donna L. Harbert Endowed Chair for Ophthalmic Research and is director of the Tony and Leona Campane Center for Excellence in Image-Guided Surgery and Advanced Imaging Research at the Cole Eye Institute. DISCLOSURES: Dr. Cakir has no relevant relationships to disclose. Dr. Ehlers reported relationships with Novartis, Zeiss, Leica, Beyeonics, Alcon, Allergan/AbbVie, Adverum, Oxurion, Roche, Allegro, Stealth, RegenxBio, Iveric bio, Boerhinger-Ingelheim, Apellis and Regeneron Pharmaceuticals. |
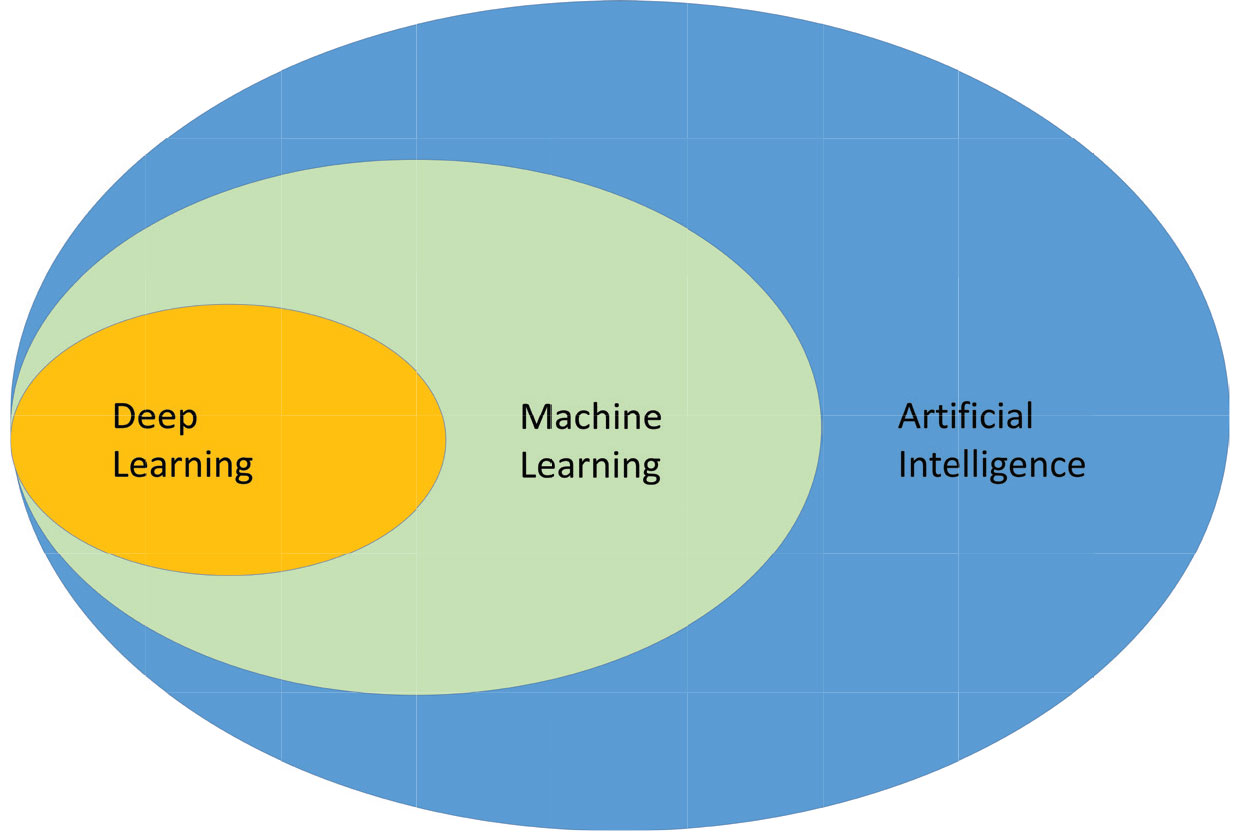
Artificial intelligence is the ability for a computer system to learn, adapt, and perform complex tasks in a way that mimics rational thinking. Machine learning is a subset of AI (Figure 1). Advancements in machine learning, the process by which algorithms and machines learn from experience, have allowed for dramatic improvements in the deployment of AI within ophthalmology and the study of retinal diseases.
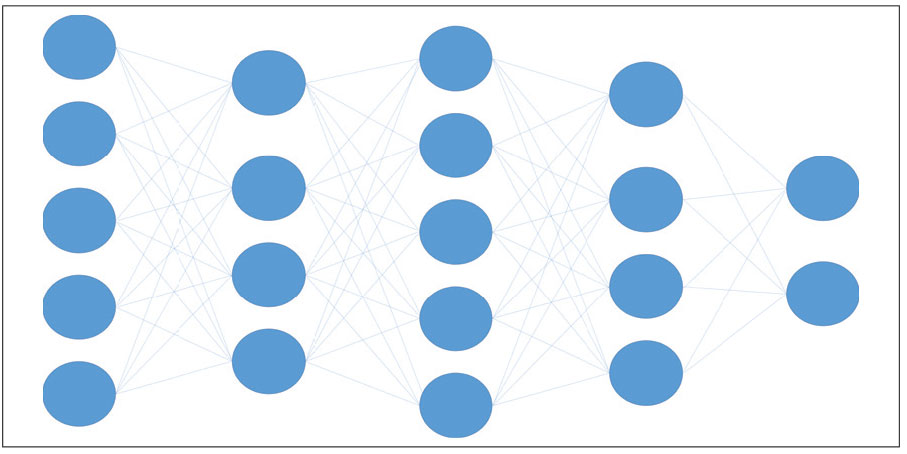
One of the significant developments in machine learning was the neural network, which is a series of numeric matrices of connected nodes that interact in such a way that allow for complex computations with hidden tunable layers that could adjust the connections between nodes (Figure 2). These networks are generally computationally taxing, but adept at capturing complex non-linear relationships. As computational power and graphics cards have exploded in capacity and potential, the size and depth of neural networks has greatly increased, resulting in deeper networks that became capable of recognizing increasingly abstract patterns.
The complexity, accuracy, and abstract pattern recognition of neural networks has become powerful enough to rival human performance on an increasingly wide and difficult range of tasks. The impressive performance of these deeply constructed neural networks has consequently created an entire technical research field dedicated to developing and understanding them, including deep learning (DL). DL algorithms are the most capable way to handle complex tasks, and they comprise the majority of AI work done today.
AI applications in retina
The retina subspecialty is particularly well-suited to AI deployment and exploration due to the ease of acquisition of medically relevant images and data. This, combined with an aging global population that’s increasingly diagnosed with chronic diseases, suggests a major opportunity for the expansion of AI research in retinal medicine.
According to a 2014 World Health Organization study, the number of AMD patients in 2040 is expected to reach 288 million globally.1 Diabetes mellitus has a global prevalence varying between 2 and 12 percent.2 In real-world studies, one of three patients with diabetes has diabetic retinopathy, and one of every three patients with DR has vision-threatening retinopathy.3
Given the large number of people affected by these diseases and the potential to better understand and diagnose these diseases using AI, it’s worthwhile to understand specific applications of AI in ophthalmology.
 |
|
Figure 1. How artificial intelligence, machine learning and deep learning relate to each other. Deep learning is a subset of machine learning, which is a subset of AI. |
AI in AMD
While there’s no Food and Drug Administration-approved treatment for dry age-related macular degeneration,4 multiple therapies in clinical trials have demonstrated promising initial results. However, as our treatment paradigms expand to include dry AMD, multiple new challenges in the detection and monitoring of this highly prevalent disease will emerge.
We’ll need risk stratification of dry AMD cases to accurately identify eyes at risk for disease progression, to predict eyes most likely to benefit from therapy and to potentially maximize clinical trial enrichment. Optimizing the precision approach to the management of dry AMD will help facilitate the treatment of patients at greatest risk for progression and help identify eyes that could benefit from therapy.
 |
|
Figure 2. A convolutional neural network (CNN) is a network architecture for deep learning that learns directly from data. A CNN, like other neural networks, is made up of an input layer, an output layer and several hidden layers in between. These layers conduct operations on the data with the goal of learning data-specific attributes. |
Performing effective, rapid risk stratification in a growing and aging population requires rapid, inexpensive and accurate diagnostics. AI-enabled systems are well-suited to address these concerns. Imaging techniques, such as optical coherence tomography, fundus autofluorescence and OCT angiography, can rapidly generate detailed data of sufficient quantities to train AI systems to identify medically useful biomarkers and automate disease characterization (Figure 3, below). Incorporating this information and automated detection into standard-of-care medical software platforms allows for scalable longitudinal monitoring, providing valuable feedback on treatment results.
Numerous imaging biomarkers have been studied in non-neovascular AMD, including intraretinal hyperreflective foci (HRF), complex drusenoid lesions (DL, i.e., heterogeneous reflectivity), subretinal drusenoid deposits (SDDs) and drusen burden.5,6 Predicting GA development and progression is a key area of research focus.7–10 A recent study found that ellipsoid zone integrity and quantitative characterization of the subretinal pigment epithelium compartments have high predictive value in the progression to subfoveal GA.11
In neovascular AMD, feature assessment and accurate characterization of fluid is important for maximizing disease control and outcomes. OCT is the gold standard for monitoring and identifying fluid features. Historically, fluid features were generally evaluated in a binary fashion (i.e., presence/absence of a specific fluid of interest).
With the advent of AI-enabled systems, extensive characterization of fluid in nAMD is now feasible, including fluid volume analysis and specific fluid type classification. Volumetric fluid characterization, fluid dynamics and exudative volatility, and volumetric quantification of subretinal fibrosis and hyperreflective material have all been established in several studies.12–14
Overall, many potential opportunities exist for the deployment and integration of AI-based tools for the management of AMD.
AI in DR
 |
|
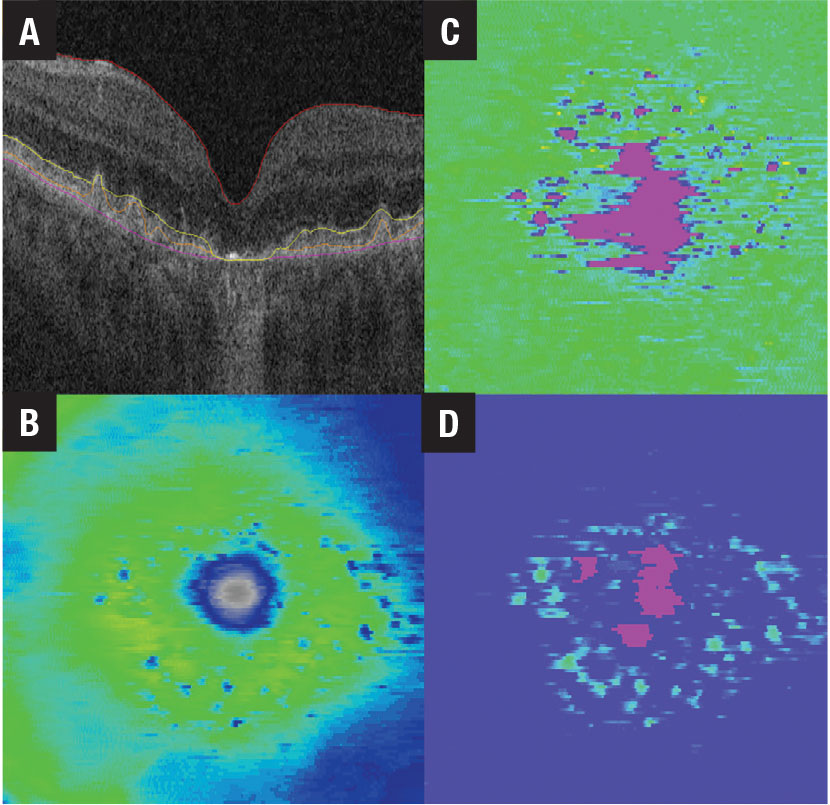
Figure 3. Retinal tissue thickness maps for a patient with non-neovascular age-related macular degeneration include A) B-scan with inner limiting membrane, retinal pigment epithelium, ellipsoid zone and Bruch’s membrane line segmentation; B) ILM-RPE thickness map; D) EZ-RPE thickness map; and D) RPE-Bruch’s membrane thickness map. |
Studies anticipate that by 2030 450 million people will have diabetes worldwide—150 million with mild DR and about 30 million with diabetic macular edema.15,16 While DR is treatable, slow and expensive screening can be a major barrier when treating large populations. AI is well positioned to make significant improvements in these screening processes while integrating into holistic platforms designed to coordinate with treatments for other complications typically resulting from diabetes.17
One example of merging diabetic retinopathy screening with AI is IDx-DR (Digital Diagnostics), the first FDA-approved autonomous device employing artificial intelligence software.18 A pivotal trial of 900 patients reported device sensitivity and specificity for diabetes detection were 87.4 and 89.5 percent, respectively.
If the software detects severe DR, the patient is referred to an eye-care professional. If the software doesn’t detect severe or moderate DR, the patient is directed to rescreen in a year. The study reported that DR screening has successfully referred individuals with DR symptoms over specific thresholds to specialized facilities.18 The idea is to reduce patient burden on tertiary centers, allowing providers to spend more time with patients who need advanced care.
While anti-VEGF therapy is widely used to treat DME, retinal specialists disagree on the ideal treatment frequency or duration, or which patients will respond, resulting in expensive and potentially more frequent treatment than needed.
 |
|
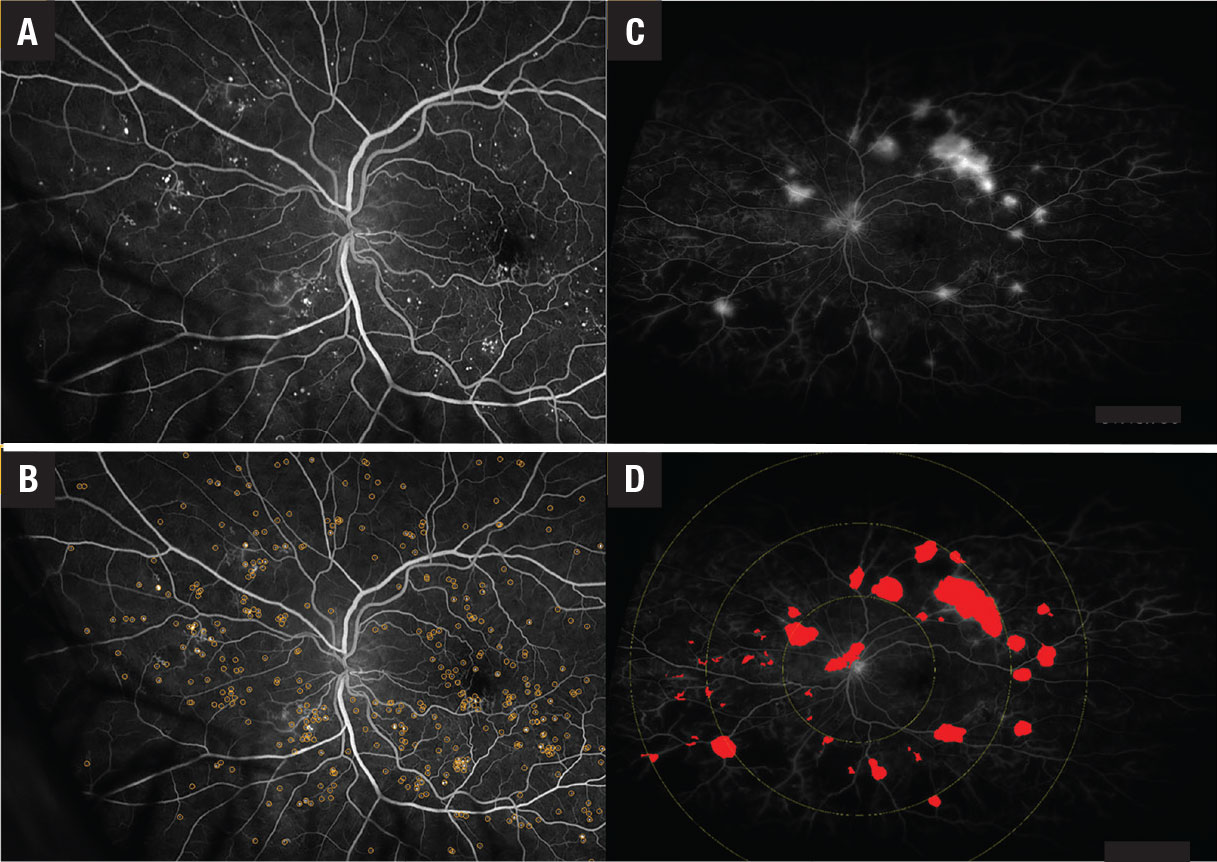
Figure 4. A,B) Early fluorescein angiography shows microaneurysms with and without annotation in a patient with diabetic retinopathy. C,D) Late ultra-widefield FA shows leakage with and without annotations in a DR patient. |
Deep-learning algorithms might be able to recognize new features undetectable by the human eye and incorporate existing complex biomarkers, such as ultra-widefield fluorescein angiography biomarkers (nonperfusion area, ischemic index, leakage and microaneurysm counts).19,20 These algorithms have also recognized the following OCT biomarkers (Figure 4):21–26
- central subfield thickness and disorganization of retinal inner layers (DRIL)
- hyperreflective foci;
- ellipsoid zone integrity;
- retinal fluid index; and
- retinal fluid volatility.
These markers have been shown to correlate with DR severity. Many of these parameters, such as quantitative UWFA parameters, including panretinal MA count, ischemia and leakage index, have been strongly associated with DR severity.20
As with AMD, the rapid proliferation of new and emerging therapies provides a unique need and opportunity for optimizing treatment decision-making using a precision-based approach. AI-enabled systems that provide insights into therapeutic response and prognosis may enhance not only screening and diagnosis, but may also help to optimize the actual treatment for a given patient.
Bottom line
AI is poised to expand dramatically in the coming years, given the growing population of patients with ophthalmic needs may overwhelm traditional methods for screening and chronic disease follow-up. AI-enabled systems may help mitigate some of these challenges by efficiently managing large amounts of data and having the unique ability to extract key features relevant to disease management, including identifying key imaging biomarkers. RS
References
1. Wong WL, Su X, Li X, et al. Global prevalence of age-related macular degeneration and disease burden projection for 2020 and 2040: A systematic review and meta-analysis. Lancet Glob Health. 2014;2:e106-116.
2. Sabanayagam C, Banu R, Chee ML, et al. Incidence and progression of diabetic retinopathy: A systematic review. Lancet Diabetes Endocrinol. 2019;7:140-149.
3. Lee R, Wong TY, Sabanayagam C. Epidemiology of diabetic retinopathy, diabetic macular edema and related vision loss. Eye Vis (Lond). 2015;2:17.
4. Handa JT, Bowes Rickman C, Dick AD, et al. A systems biology approach towards understanding and treating non-neovascular age-related macular degeneration. Nat Commun. 2019;10:3347.
5. Toth CA, Tai V, Chiu SJ, et al. Linking OCT, angiographic, and photographic lesion components in neovascular age-related macular degeneration. Ophthalmol Retina. 2018;2:481-493.
6. Nassisi M, Lei J, Abdelfattah NS, et al. OCT risk factors for development of late age-related macular degeneration in the fellow eyes of patients enrolled in the HARBOR study. Ophthalmology. 2019;126:1667-1674.
7. Bogunovic H, Montuoro A, Baratsits M, et al. Machine learning of the progression of intermediate age-related macular degeneration based on OCT imaging. Invest Ophthalmol Vis Sci. 2017;58:BIO141-BIO150.
8. Hirabayashi K, Yu HJ, Wakatsuki Y, Marion KM, Wykoff CC, Sadda SR. Optical coherence tomography risk factors for development of atrophy in eyes with intermediate age-related macular degeneration. Ophthalmol Retina. 2022;S2468-6530 (22) 00486-9.
9. Riedl S, Vogl WD, Mai J, et al. The effect of pegcetacoplan treatment on photoreceptor maintenance in geographic atrophy monitored by artificial intelligence-based OCT analysis. Ophthalmol Retina. 2022;S2468-6530 (22)00285-8.
10. Niu S, de Sisternes L, Chen Q, Rubin DL, Leng T. Fully automated prediction of geographic atrophy growth using quantitative spectral-domain optical coherence tomography biomarkers. Ophthalmology. 2016;123:1737-1750.
11. Sarici K, Abraham JR, Sevgi DD, et al. Risk classification for progression to subfoveal geographic atrophy in dry age-related macular degeneration using machine learning-enabled outer retinal feature extraction. Ophthalmic Surg Lasers Imaging Retina .2022;53:31-39.
12. Ehlers JP, Patel N, Kaiser PK, et al. The association of fluid volatility with subretinal hyperreflective material and ellipsoid zone integrity in neovascular AMD. Invest Ophthalmol Vis Sci. 2022;63:17.
13. Ehlers JP, Zahid R, Kaiser PK, et al. Longitudinal assessment of ellipsoid zone integrity, subretinal hyperreflective material, and subretinal pigment epithelium disease in neovascular age-related macular degeneration. Ophthalmol Retina. 2021;5:1204-1213.
14. Ehlers JP, Clark J, Uchida A, et al. Longitudinal higher-order OCT assessment of quantitative fluid dynamics and the total retinal fluid index in neovascular AMD. Transl Vis Sci Technol. 2021;10:29.
15. Shaw JE, Sicree RA, Zimmet PZ. Global estimates of the prevalence of diabetes for 2010 and 2030. Diabetes Res Clin Pract. 2010;87:4-14.
16. Yau JW, Rogers SL, Kawasaki R, et al. Global prevalence and major risk factors of diabetic retinopathy. Diabetes Care. 2012;35:556-564.
17. Williams R, Karuranga S, Malanda B, et al. Global and regional estimates and projections of diabetes-related health expenditure: Results from the International Diabetes Federation Diabetes Atlas, 9th edition. Diabetes Res Clin Pract. 2020;162:108072.
18. Abràmoff MD, Lavin PT, Birch M, Shah N, Folk JC. Pivotal trial of an autonomous AI-based diagnostic system for detection of diabetic retinopathy in primary care offices. NPJ Digit Med. 2018;1:39.
19. Jiang AC, Srivastava SK, Hu M, et al. Quantitative ultra-widefield angiographic features and associations with diabetic macular edema. Ophthalmol Retina. 2020;4:49-56.
20. Ehlers JP, Jiang AC, Boss JD, et al. Quantitative ultra-widefield angiography and diabetic retinopathy severity: An assessment of panretinal leakage index, ischemic index and microaneurysm count. Ophthalmology. 2019;126:1527-1532.
21. Ehlers JP, Uchida A, Hu M, et al. Higher-order assessment of OCT in diabetic macular edema from the VISTA Study: Ellipsoid zone dynamics and the Retinal Fluid Index. Ophthalmol Retina. 2019;3:1056-1066.
22. Ehlers JP, Uchida A, Sevgi DD, et al. Retinal fluid volatility associated with interval tolerance and visual outcomes in diabetic macular edema in the VISTA Phase III trial. Am J Ophthalmol. 2021;224:217-227.
23. Vujosevic S, Midena E. Retinal layers changes in human preclinical and early clinical diabetic retinopathy support early retinal neuronal and Müller cells alterations. J Diabetes Res. 2013;2013:905058.
24. Shi R, Guo Z, Wang F, Li R, Zhao L, Lin R. Alterations in retinal nerve fiber layer thickness in early stages of diabetic retinopathy and potential risk factors. Curr Eye Res. 2018;43:244-253.
25. Deák GG, Schmidt-Erfurth UM, Jampol LM. Correlation of central retinal thickness and visual acuity in diabetic macular edema. JAMA Ophthalmol. 2018;136:1215-1216.
26. Joltikov KA, Sesi CA, de Castro VM, et al. Disorganization of retinal inner layers (DRIL) and neuroretinal dysfunction in early diabetic retinopathy. Invest Ophthalmol Vis Sci. 2018;59:5481-5486.



