| ABOUT THE AUTHOR |
 Dr. Witkin is an assistant Dr. Witkin is an assistant professor of ophthalmology and director of Clinical Research at the New England Eye Center at Tufts Medical Center, Boston. |
| Disclosures: Dr. Witkin has no financial relationships to disclose. |
Ophthalmologists have widely embraced prophylactic intracameral vancomycin during routine cataract surgery to reduce the risk of postoperative endophthalmitis, with excellent results, but this practice has not been without controversy and debate. A large, retrospective study suggested that prophylactic intracameral vancomycin did not reduce the incidence of endophthalmitis.1 Other authors have suggested that routine prophylactic use may increase the risk of medication toxicity and drug contamination, and promote vancomycin resistance.1-4 The Centers for Disease Control & Prevention specifically recommended avoiding vancomycin for routine prophylactic use in surgery to prevent the spread of vancomycin resistance.4
More recently, a rare but devastating complication has been associated with prophylactic intraocular vancomycin use in cataract surgery, although the cause of the disease is not definitively known.5 My colleagues and I published a series of 11 eyes of six patients who all had a similar appearance of retinal vascular occlusion and hemorrhage with subsequent severe vision loss in many cases, which we termed hemorrhagic occlusive retinal vasculitis (HORV). The following is a case of a woman who experienced severe vision loss and neovascular glaucoma after cataract surgery, along with a review of the literature. A sidebar summarizes recommendations to avoid HORV (page 29).
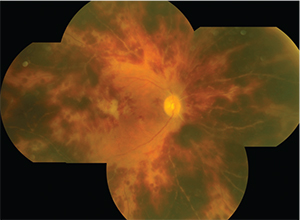 |
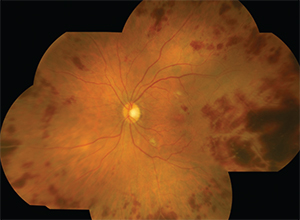
| Figure 1. The patient’s right eye (above) 18 days after cataract surgery showed diffuse microvascular occlusion and extensive intraretinal hemorrhages worse than the left eye (below), imaged 11 days after surgery. |
 |
Admission After Intracameral Vancomycin
In the summer of 2014, a 66-year-old woman had sequential and uneventful cataract surgery at an ambulatory surgery center, first in the right eye and one week later in the left. In addition to preservative-free lidocaine and viscoelastic, prophylactic intracameral vancomycin (1 mg/0.1 ml) was used during both surgeries. On postoperative day one, the uncorrected visual acuity was 20/25 in each eye and the patient was happy.
However, 10 days after surgery she first noticed blurred vision in the right eye. The cataract surgeon noted diffuse retinal hemorrhages in the right eye. She was referred to an outside retina specialist.
The retina specialist initiated oral valacyclovir 1,000 mg t.i.d. for presumed acute retinal necrosis. One week later, the patient noticed loss of vision in the left eye, as the right eye continued to deteriorate. Similar retinal findings were seen in the left eye, and the patient was referred to our service at the New England Eye Center of Tufts Medical Center.
On presentation to the clinic 18 days after the initial surgery, visual acuity was counting fingers in the right eye and 20/60 in the left. Mild cell and fibrin were noted in the anterior chamber with rare vitreous cell in both eyes. Intraocular pressure was 24 mmHg in both eyes. Most strikingly, the retinal examination showed diffuse microvascular occlusion and extensive intraretinal hemorrhages in both eyes, worse in the right eye than the left (Figure 1).
Fluorescein angiography showed stark nonperfusion at the border of active vasculitis (Figure 2). The patient was admitted for further evaluation.
Inpatient Treatment
Inpatient treatment included intravitreal ganciclovir and foscarnet bilaterally, high-dose intravenous corticosteroids and oral valacyclovir for the possibility of acute retinal necrosis. Extensive ocular and systemic testing for hematologic, rheumatologic, infectious and neoplastic causes was negative. After 10 days, she was discharged on oral prednisone and valacyclovir, which we tapered over two months.
One month after stopping oral prednisone, hypersensitivity skin testing was negative for reactions to vancomycin, sodium hyaluronate or lidocaine.
Three months after she first came to our clinic, the patient had developed neovascular glaucoma in the right eye. Despite intravitreal bevacizumab (Avastin, Genentech) injections, visual acuity in the right eye deteriorated to no light perception. She underwent a trans-scleral cyclophotocoagulation to control intraocular pressure. The left eye remained unchanged, with a resultant visual acuity of 20/200 at one-year follow-up. She received intravitreal ranibizumab (Lucentis, Genentech) and prophylactic panretinal photocoagulation in the left eye to prevent neovascular glaucoma.
Identifying Similar Cases
Around the same time as the patient described above was admitted, Laura Nicholson, MD, and colleagues at Massachusetts Eye and Ear Infirmary in Boston published a report demonstrating an identical disease in two other patients.6 After we discussed these cases with the authors of that report and other colleagues, a total of 11 eyes of six patients were collected with an identical appearance to our case, and the authors termed the condition HORV.
| Take-home Point Intracameral vancomycin during routine cataract surgery has gained acceptance as prophylaxis against postoperative endophthalmitis. Despite overall excellent results with this approach, a rare but visually devastating complication, hemorrhagic occlusive retinal vasculitis (HORV), has been reported. This article discusses a series of 11 eyes and offers recommendations for preventing and managing HORV. |
All 11 eyes had the following characteristics in common:
• Occurrence after otherwise uncomplicated cataract surgery.
• Use of prophylactic intraocular vancomycin during surgery.
• Delayed onset of painless visual loss between one and 14 days after surgery.
• Retinal vascular occlusions associated with hemorrhages in non-perfused areas.
• Relatively mild anterior chamber and vitreous inflammation.
• Poor visual results were common, often complicated by neovascular glaucoma.
• Negative extensive intraocular and systemic workups.
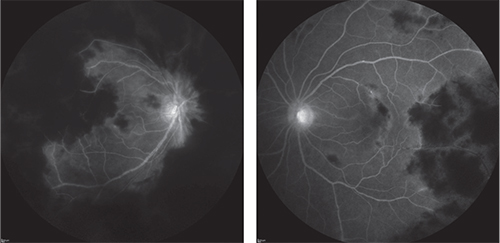 |
| Figure 2. Fluorescein angiogram of the right (left) and left eyes demonstrates complete occlusion of retinal blood flow in regions of retinal hemorrhages as well as leakage from retinal venules and the optic nerve. |
Because the onset of disease was delayed, HORV does not appear to be related to the so-called “red man” syndrome, which is a mast cell-mediated skin reaction that occurs immediately upon injection of intravenous vancomycin and resolves immediately after discontinuation of medication infusion.7 The reaction in HORV rather appears more similar to rare reports of leukocytoclastic vasculitis and Henoch-Schönlein purpura secondary to vancomycin, both of which are mediated by antibody/antigen complex deposition causing small-vessel vasculitis occurring one to two weeks after initiation of intravenous vancomycin.8–10
Not Drug Toxicity
HORV likely does not represent a toxic reaction; onset was delayed up to 14 days after surgery, and some of the patients developed the same condition in the fellow eye even when the second cataract surgery was delayed by months or years. In addition, other eyes operated on the same day as those that developed HORV did not have any similar complications despite being operated on by the same cataract surgeon using the same technique, including intracameral vancomycin from the same batch.
| Recommendations to Prevent and Manage Hemorrhagic Occlusive Retinal Vasculitis Although the precise etiology of hemorrhagic occlusive retinal vasculitis (HORV) remains unproven, the most likely explanation is an immune reaction to intraocular vancomycin. To prevent HORV, we suggest the following: • Consider avoiding prophylactic intraocular vancomycin during eye surgery. • Consider waiting at least two weeks before operating on the fellow eye. • Suspect HORV if a patient describes delayed onset visual loss after eye surgery. • Delay or avoid fellow eye surgery if HORV presents in the first eye. • Avoid intraocular adjuvant medications if HORV develops in the first eye. Management recommendations for HORV are as follows: • Consider high-dose systemic and topical corticosteroids. • Consider early anti-VEGF treatment. • Early panretinal photocoagulation to non-perfused regions. • Consider systemic antiviral therapy if acute retinal necrosis is suspected. • Avoid intravitreal vancomycin if possible. • Consider ocular and systemic workup for masquerade syndromes. The American Society of Retinal Specialists (ASRS) is collecting information about suspected cases of HORV. If you have encountered a similar case, report the case details to the ASRS Research and Safety in Therapeutics Committee at this link: http://goo.gl/vst6yR |
If HORV is due to an immune reaction to vancomycin, it is likely extremely rare. In this series, all 11 eyes received intraocular vancomycin at the dose 1 mg/0.1 ml, which has been given intravitreally for many years for treatment of endophthalmitis without a known toxic reaction to the medication. In addition, since the European Society of Cataract and Refractive Surgeons report in 2007,11 tens of thousands of eyes have received intracameral vancomycin after cataract surgery, without emergence of an epidemic of HORV. However, it is possible that there may be some cases of HORV that are less severe and have not been detected or reported. A recent case report demonstrated a milder case of HORV that spontaneously resolved without visual sequelae.12
A Poor Prognosis
The natural history of HORV can be visually devastating, because it carries is a high risk of neovascular glaucoma. In the cases we discuss here, final visual acuity was 20/100 or worse in eight of 11 eyes. Seven of 11 eyes developed neovascular glaucoma; four of these eyes had visual outcomes of NLP vision. The outcomes of HORV are particularly disturbing in patients with poor visual outcomes in both eyes due to bilateral HORV.
All patients in this series received high-dose systemic and topical steroids, which may help slow the progression of the disease. Intravitreal anti-VEGF therapy and PRP seemed to delay or prevent the onset of neovascular glaucoma in the eyes with better outcomes. Of note, four eyes in this series received intravitreal vancomycin (and ceftazidime) for treatment of possible bacterial endophthalmitis; these eyes seemed to have particularly poor outcomes, suggesting that the immune reaction may have progressed further after introduction of a second dose of intraocular vancomycin. RS
| How Intracameral Vancomycin Prophylaxis Emerged In 2007, the European Society of Cataract and Refractive Surgeons (ESCRS) published results from a large prospective, randomized, multicenter study demonstrating that prophylactic intracameral cefuroxime injection (1 mg/0.1 ml) given at the end of cataract surgery reduced the risk of postoperative endophthalmitis fivefold.11 Since that report, many ophthalmic surgeons have advocated routine use of intracameral antibiotics during cataract surgery.13–15 Both the 2011 American Academy of Ophthalmology Cataract Preferred Practice Pattern Guidelines and a 2011 American Society of Cataract and Refractive Surgeons cataract clinical committee review of endophthalmitis prevention noted that the evidence supporting direct intracameral injection was stronger than for any other method of antibiotic prophylaxis.15,16 The percentage of cataract surgeons using prophylactic intracameral antibiotics routinely during cataract surgery more than doubled from 15 percent in 2007 to 36 percent in 2014.13 In this country, surgeons have mostly used off-label moxifloxacin or vancomycin, usually mixed by the operating room staff the day of surgery, because the commercial formulation of intraocular cefuroxime used in the ESCRS study is not available in the United States.13 Many studies have supported the safety of these off-label medications for intracameral use.1,17,18 A recent study, although retrospective, reported a reduction in endophthalmitis rates with prophylactic intracameral vancomycin during cataract surgery with no cases of medication toxicity in almost 10,000 eyes.18 |
REFERENCES
1. Rudnisky CJ, Wan D, Weis E. Antibiotic choice for the prophylaxis of post-cataract extraction endophthalmitis. Ophthalmology. 2014;121:835–841.
2. Gupta A. Re: Rudnisky et al: Antibiotic choice for the prophylaxis of post-cataract extraction endophthalmitis. (Ophthalmology. 2014;121:835e41). Ophthalmology. 2014;121:e51.
3. Schimel AM, Alfonso EC, Flynn HW Jr. Endophthalmitis prophylaxis for cataract surgery: are intracameral antibiotics necessary? JAMA Ophthalmol. 2014;132:1269–1270.
4. Centers for Disease Control and Prevention. Recommendations for preventing the spread of vancomycin resistance: recommendations of the Hospital Infection Control Practices Advisory Committee (HICPAC). MWWR Morb Mortal Wkly Report. 1995;44:782.
5. Witkin AJ, Shah AR, Engstrom RE, et al. Postoperative hemorrhagic occlusive retinal vasculitis: expanding the clinical spectrum and possible association with vancomycin. Ophthalmology. 2015;122:1438-1451.
6. Nicholson LB, Kim BT, Jardón J, et al. Severe bilateral ischemic retinal vasculitis following cataract surgery. Ophthalmic Surg Lasers Imaging Retina. 2014;45:338-342.
7. Wallace MR, Mascola JR, Oldfield EC. Red man syndrome: incidence, etiology, and prophylaxis. J Infect Dis. 1991;164:1180–1185.
8. Heijnen EB, Bentala M, van der Meer NJ. Purpura in a patient receiving vancomycin: a leukoclastic vasculitis? J Cardiothorac Vasc Anesth. 2011;25:390–391.
9. Pongruangporn M, Ritchie DJ, Lu D, Marschall J. Vancomycin-associated leukocytoclastic vasculitis. Case Rep Infect Dis. 2011;2011:356370.
10. Bataille S, Daumas A, Tasei AM, et al. Vancomycin-induced Henoch-Schönlein purpura: a case report. J Med Case Rep. 2012;6:106.
11. Endophthalmitis Study Group, European Society of Cataract & Refractive Surgeons. Prophylaxis of postoperative endophthalmitis following cataract surgery: results of the ESCRS multicenter study and identification of risk factors. J Cataract Refract Surg. 2007;33:978–988.
12. Lenci LT, Chin EK, Carter C, Russell SR, Almeida DR. Ischemic retinal vasculitis associated with cataract surgery and intracameral vancomycin. Case Rep Ophthalmol Med. 2015;2015:683194. [Epub 2015 Nov 5.]
13. Chang DF, Braga-Mele R, Henderson BA, Mamalis N, Vasavada A; ASCRS Cataract Clinical Committee. Antibiotic prophylaxis of postoperative endophthalmitis after cataract surgery: Results of the 2014 ASCRS member survey. J Cataract Refract Surg. 2015;41:1300-1305.
14. Behndig A, Cochener B, Güell JL, et al. Endophthalmitis prophylaxis in cataract surgery: overview of current practice patterns in 9 European countries. J Cataract Refract Surg. 2013;39:1421–1431.
15. Packer M, Chang DF, Dewey SH, et al. Prevention, diagnosis, and management of acute postoperative bacterial endophthalmitis. J Cataract Refract Surg. 2011;37:1699–1714.
16. American Academy of Ophthalmology. Cataract in the Adult Eye; Preferred Practice Pattern. San Francisco, CA, American Academy of Ophthalmology, 2011. Available at: http://www.aao.org/preferred-practice-pattern/cataract-in-adult-eye-ppp--october-2011. Accessed February 19, 2016.
17. Braga-Mele R, Chang DF, Henderson BA, Mamalis N, Talley-Rostov A, Vasavada A; for the ASCRS Clinical Cataract Committee. Intracameral antibiotics: safety, efficacy, and preparation. J Cataract Refract Surg. 2014;40:2134–2142.
18. Rush SW, Vu D, Rush RB. The safety and efficacy of routine administration of intracameral vancomycin during cataract surgery. J Ophthalmol. 2015;2015:813697. [Epub 2015
Nov 4].



