 |
 |
 |
 |
Gene therapy for the treatment of ocular disease has undergone significant development in recent years, particularly in the area of inherited retinal disease.1 Ocular disease is a well-suited target for possible genetic therapies for a variety of reasons: the relative immune isolation of the eye; the availability of the contralateral eye as a control; the relative ease of treatment delivery; its enclosed structure; and the numerous methods of noninvasive outcome measures available.1 Increasingly, age-related macular degeneration is emerging as a desirable, if somewhat complex, target for gene therapy.
Reasons for this go beyond AMD being a leading cause of blindness worldwide, although it’s projected to affect approximately 196 million people in 2020.2
Anti-VEGF intravitreal injections are far from perfect. The need for frequent visits and repeated injections is associated with a substantial burden on patients, family members and the health-care system.1,3
Patient compliance with the required treatment schedules can be an issue. The cumulative risk of repeated intravitreal injections isn’t insignificant; they include the risk of endophthalmitis, intraocular hemorrhage, retinal detachment, uveitis and ocular hypertension.4
Furthermore, anti-VEGF therapy isn't always efficacious. In the Comparison of Age-Related Macular Degeneration Treatments Trials (CATT), 34.1 percent of the monthly ranibizumab (Lucentis, Roche/Genentech) arm and 31.7 percent of the monthly bevacizumab (Avastin, Roche/Genentech) arm showed either no significant improvement or worsening of best- corrected visual acuity at one year.5 While anti-VEGF injections offer a drastic improvement over previous options, newer therapies that offer higher efficacy or reduce the injection burden would certainly be a benefit.
A complex genetic profile
Compared to monogenic inherited retinal diseases, however, the pathogenesis of AMD is thought to be complex, with multiple genetic factors and environmental exposures contributing to the disease.3 While multiple genes, such as CFH, CFB and ApoE, have been associated with the disease, our understanding of the genetic pathology of AMD remains incomplete. As such, the American Academy of Ophthalmology recommends avoiding routine genetic testing.6 While the complex nature of AMD will likely require a multimodal approach, the potential role for gene therapy is strong, as evidenced by numerous recent and ongoing studies.7
 |
Gene therapies require a method for delivery to the target cells. One popular method is the use of viral vectors, particularly adeno-associated virus (AAV).8 This virus is attractive due to its lack of pathogenicity, multiple available serotypes, strong persistence despite a low-integration frequency and tendency to induce minimal host immune response, reducing the risk of destruction of the virus or transduced cells.8
Gene therapy and the FLT1 gene
The FLT1 gene encodes a member of the vascular endothelial growth factor
receptor family, the tyrosine kinase
receptors. These proteins bind to VEGF-A, VEGF-B and placental growth factor (PLGF), acting as natural VEGF inhibitors.10 AAV2-sFLT01 (Sanofi/Genzyme) uses an AAV2 vector to deliver a gene that encodes the VEGF-neutralizing soluble Fms-related kinase-1 (sFLT-1).10 It’s given via intravitreal
 |
injection.
A Phase I open-label, dose-escalation trial in patients with wet AMD, subretinal fibrosis and vision worse than 20/100 reported no systemic side effects and
ocular inflammation in only the highest-dose group.10
The trial demonstrated an anti-permeability effect, but also noted variability of expression with some, but not all, subjects showing response with reduction of intra- and subretinal fluid.8,10 The variability has been attributed to pre-existing antibodies to AAV in some patients, which some authors have hypothesized limit transduction of target cells and thereby limit production of the desired protein.10
Similarly, a Phase I randomized controlled trial of subretinal injection of rAAV sFLT-1 reported that four of six patients in the treatment arm didn’t require any anti-VEGF rescue injections.11 Although it was a single-center study of only nine patients with relatively short follow-up, the trial demonstrated safety of the therapy. The results were maintained at 36 months.12 Combined Phase I/IIa three-year results, comparing 24 treated patients and 13 controls, similarly showed a good safety profile.13 However, because the study was designed to assess safety, it examined elderly patients with advanced disease and was unable to draw any significant conclusions regarding efficacy.13
 |
RGX-314: AAV8, subretinal delivery
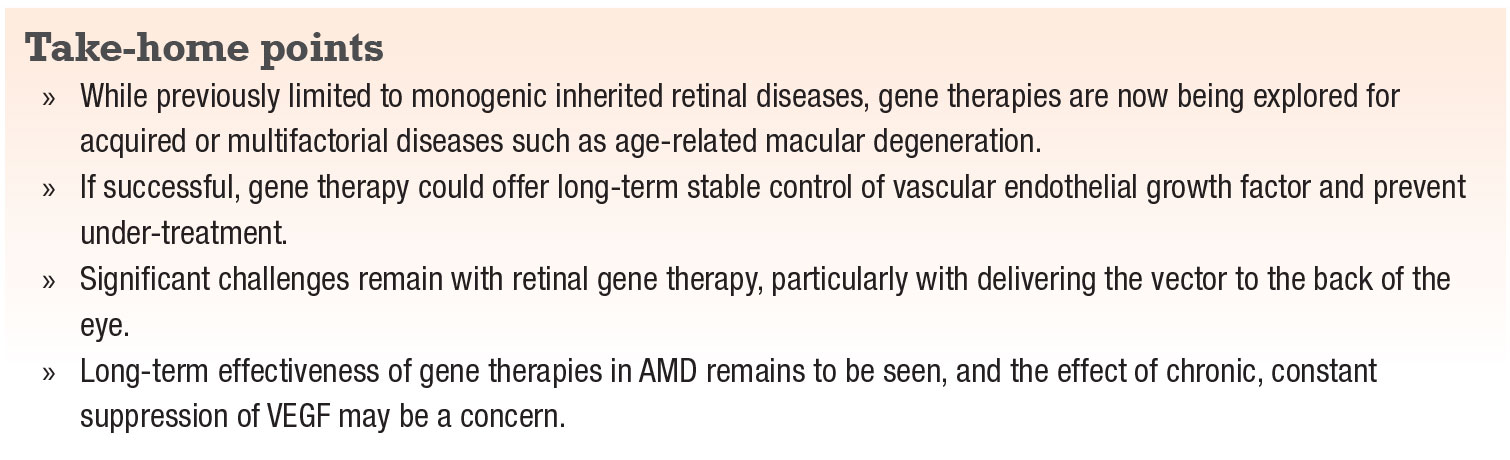
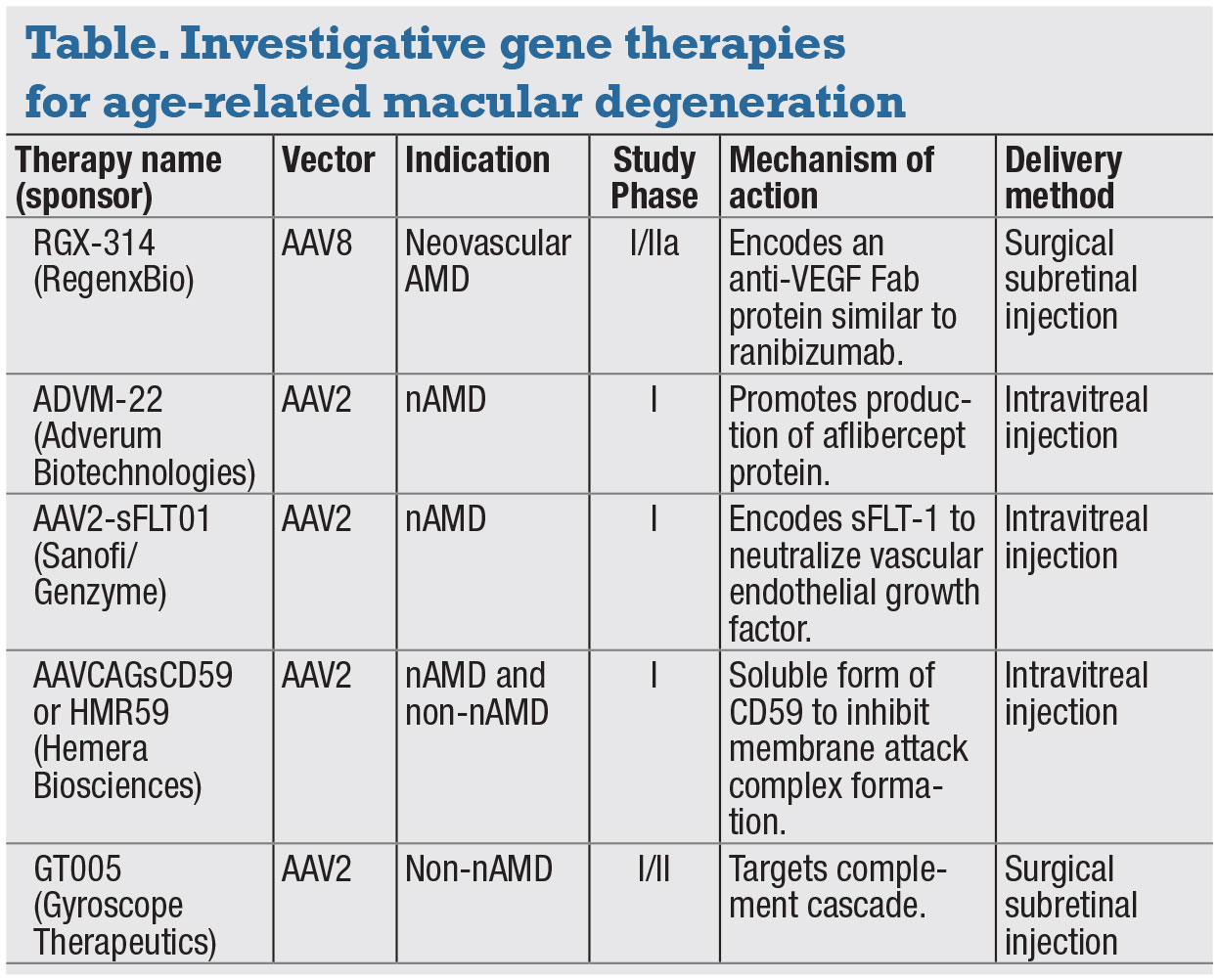
One gene therapy that recently completed Phase I/IIa trials for the treatment of wet AMD is RGX-314 (RegenxBio).7,9 It consists of an AAV serotype 8 vector delivering a gene that encodes a monoclonal antibody fragment that binds VEGF-A (Figure 1, page 17).8,9 The treatment requires a pars-plana vitrectomy to deliver the vector via subretinal injection, with the goal of transducing the retinal pigment epithelium.8,9
It’s designed to induce the sustained production of a VEGF-binding antibody within the retina, leading to wet AMD disease control.8,9 In the clinical trial, each patient received one treatment and was monitored for 12 months. The treatment appeared safe and reduced the need for other anti-VEGF treatments for more than one year after administration.8,9 Researchers are preparing to start Phase IIb trials of RGX-314.3
ADVM-022 in-office gene therapy
 |
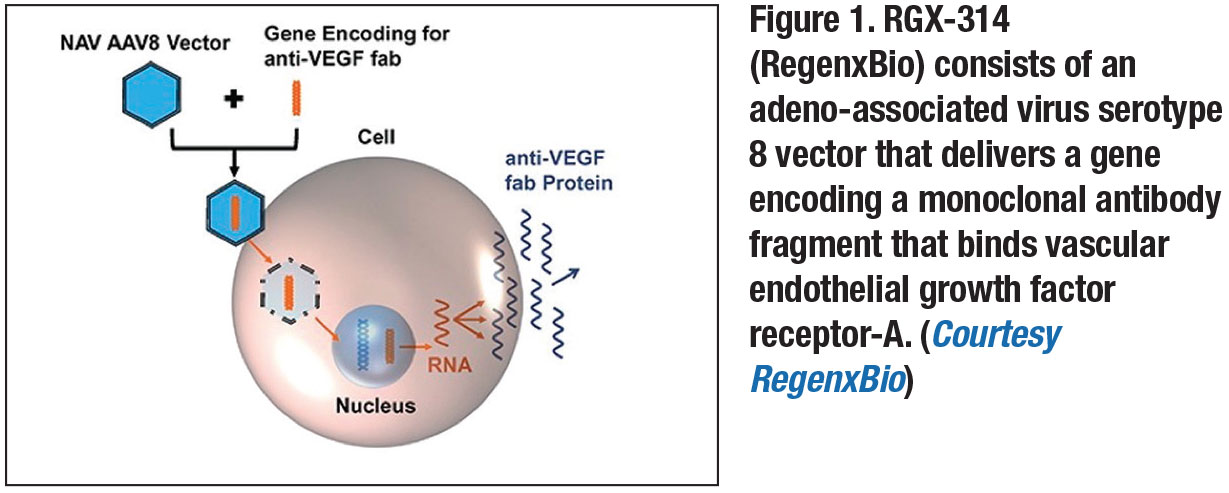
ADVM-022 (Adverum Biotechnologies) is a novel recombinant AAV optimized for intravitreal administration and more robust protein expression.14 Compared to previous modalities, it utilizes the AAV2.7m8 capsid, which has highly efficient retinal transduction, and is designed to result in strong expression of the aflibercept protein.14 It can be administered via an intravitreal injection in an office setting (Figure 2, page 17).14
The OPTIC trial is an ongoing two-year, multicenter, prospective Phase I study evaluating the safety and tolerability of ADVM-022.15 A single intravitreal injection is given seven to 14 days after a screening aflibercept injection (Eylea, Regeneron Pharmaceuticals) with a concurrent 13-day topical or oral corticosteroid course for control of inflammation. Szilard Kiss, MD, reported 24-week data at the Retina Society in September 2019 that showed a good safety profile, with mild to moderate inflammation as the only adverse event noted in all patients—although the inflammation was controlled with corticosteroids. Efficacy at 24 weeks seemed promising, with no patients needing rescue injections of anti-VEGF.
Non-neovascular AMD gene therapy
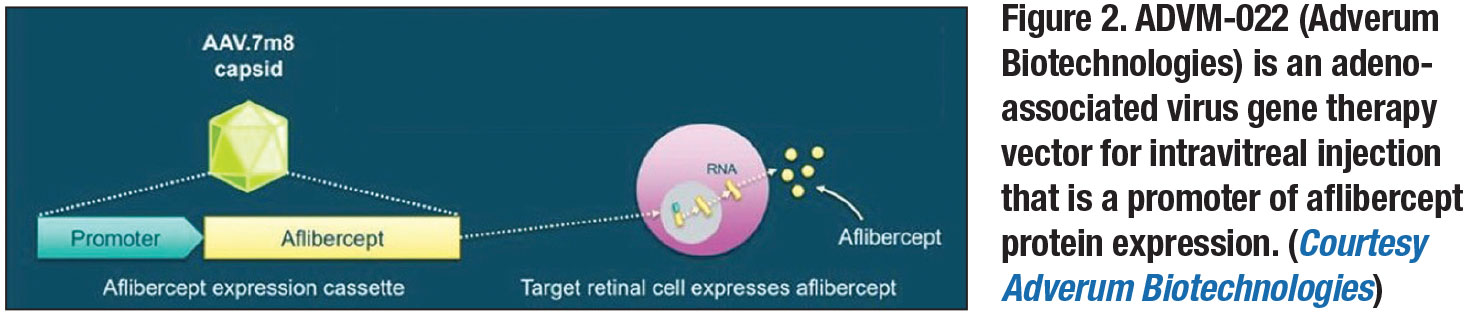
Another gene therapy for AMD, HMR59 (Hemera Biosciences), or AAVCAGsCD59, is designed to affect the complement pathway via inhibition of its end product, the membrane attack complex (MAC). 8 Several studies have linked the complement pathway and MAC formation with AMD—with MAC-mediated release of growth factors thought to contribute to choroidal neovascularization and conversion to wet AMD.8,16
Administered as a single intravitreal injection, HMR59 uses an AAV2 vector and is designed to lead to production of a form of the CD59 protein, which binds the incomplete MAC and prevents binding of the C9 proteins required to complete the complex.8 A Phase I trial assessing it in treatment-naïve wet AMD patients is currently underway (Figure 3).8,17
Because it affects the complement cascade, HMR59 also has the potential to treat dry AMD,8 which could be impactful given the lack of effective treatments, particularly for advanced dry AMD with geographic atrophy.3 Given this potential, HMR59 is also the subject of a Phase I trial for the treatment of advanced dry AMD, and early results have been promising.17
Another gene therapy currently in trials is GT005 (Gyroscope Therapeutics). Similar to HMR59, it uses an AAV vector and is designed to control complement activation.18 It's delivered via subretinal injection. Current Phase I/II trials are evaluating its effectiveness in advanced dry AMD with macular atrophy. 18
 |
Challenges of gene therapy
Despite the promise of gene therapy, significant challenges remain, particularly with delivering the vector. Most research utilizes AAV vectors, and while they have many advantages, concerns have persisted over their ability to effectively transduce cells, particularly when given intravitreally.8 Subretinal delivery may be more effective, but has been associated with higher risks, such as damage to the retina and cataract formation.8,14 The current capacity of AAV vectors is also limited, at approximately 4.7 kb.8
Long-term effectiveness of these therapies remains to be seen, and the effect of chronic, constant suppression of VEGF is a concern.8 Finally, given the difficulty of manufacturing these treatments, the price of gene therapy remains high. If future AMD therapies have similar costs, it may place a substantial burden on patients and the health-care system.
Bottom line
Gene therapy may have a substantial impact in AMD due to the high prevalence of the disease, the lack of effective treatments for advanced dry AMD and the treatment burden of wet AMD. Such multifactorial, non-monogenic diseases will likely warrant a combination of therapies, and novel targets and pathways of treatment will need to be identified. If successful, gene therapy could offer long-term stable VEGF control and prevent undertreatment from patient non-compliance in AMD. RS
REFERENCES
1. Ramlogan‐Steel CA, Murali A, Andrzejewski S, Dhungel B, Steel JC, Layton CJ. Gene therapy and the adeno-associated virus in the treatment of genetic and acquired ophthalmic diseases in humans: Trials, future directions and safety considerations. Clin Exp Ophthalmol. 2019;47:521-536.
2. Pennington KL, DeAngelis MM. Epidemiology of age-related macular degeneration (AMD): Associations with cardiovascular disease phenotypes and lipid factors. Eye Vis (Lond). 2016;3:34.
3. Lim LS, Mitchell P, Seddon JM, Holz FG, Wong TY. Age-related macular degeneration. Lancet. 2012;379:1728-1738.
4. Jager RD, Aiello LP, Patel SC, Cunningham ET. Risks of intravitreous injection: A comprehensive review: Retina. 2004;24:676-698.
5. The CATT Research Group. Ranibizumab and bevacizumab for neovascular age-related macular degeneration. N Engl J Med. 2011;364:1897-1908.
6. Stone EM. Genetic testing for age-related macular degeneration: Not indicated now. JAMA Ophthalmol. 2015;133:598-600.
7. Sengillo J, Justus S, Cabral T, Tsang S. Correction of monogenic and common retinal disorders with gene therapy. Genes (Basel). 2017;8:E53.
8. Bordet T, Behar-Cohen F. Ocular gene therapies in clinical practice: Viral vectors and nonviral alternatives. Drug Disc Today. 2019;24:1685-1693.
9. Liu Y, Fortmann SD, Shen J, et al. AAV8-antiVEGFfab ocular gene transfer for neovascular age-related macular degeneration. Mol Ther. 2018;26:542-549.
10. Heier JS, Kherani S, Desai S, et al. Intravitreous injection of AAV2-sFLT01 in patients with advanced neovascular age-related macular degeneration: A phase 1, open-label trial. Lancet. 2017;390:50-61.
11. Rakoczy EP, Lai C-M, Magno AL, et al. Gene therapy with recombinant adeno-associated vectors for neovascular age-related macular degeneration: 1 year follow-up of a Phase 1 randomised clinical trial. Lancet. 2015;386:2395-2403.
12. Constable IJ, Lai C-M, Magno AL, et al. Gene therapy in neovascular age-related macular degeneration: Three-year follow-up of a phase 1 randomized dose escalation trial. Am J Ophthalmol. 2017;177:150-158.
13. Rakoczy EP, Magno AL, Lai C-M, et al. Three-year follow-up of Phase 1 and 2a rAAV.sFLT-1 subretinal gene therapy trials for exudative age-related macular degeneration. Am J Ophthalmol. 2019;204:113-123.
14. Grishanin R, Vuillemenot B, Sharma P, et al. Preclinical evaluation of ADVM-022, a novel gene therapy approach to treating wet age-related macular degeneration. Mol Ther. 2019;27:118-129.
15. ADVM-022 intravitreal gene therapy for wet AMD (OPTIC). ClinicalTrials.gov identifier: NCT 03748784. https://clinicaltrials.gov/ct2/show/NCT03748784. Accessed February 8, 2020.
16. Bora PS, Sohn J-H, Cruz JMC, et al. Role of complement and complement membrane attack complex in laser-induced choroidal neovascularization. J Immunol. 2005;174:491-497.
17. Puliafito CA, Wykoff CC. Looking ahead in retinal disease management: Highlights of the 2019 angiogenesis, exudation and degeneration symposium. Int J Retin Vitr. 2019;5:22.
18. First in human study to evaluate the safety and efficacy of GT005 administered in subjects with dry AMD. ClinicalTrials.gov identifier: NCT 03846193. https://clinicaltrials.gov/ct2/show/NCT03846193. Accessed February 6, 2020.



