Take-home points
|
 |
|
Bios Dr. Rafieetary is a second-year retina fellow at the Charles Retina Institute in Germantown, Tennessee. |
In 2017 voretigene neparvovec became the first retina gene therapy to receive Food and Drug Administration approval. Voretigene neparvovec is an adeno-associated virus that delivers a functional copy of the RPE65 gene subretinally to treat Leber congenital amaurosis.
Since voretigene neparvovec (Luxturna, Spark Therapeutics) became available, active research has exploded into other modes of gene therapy delivery as well as indications beyond inherited retinal disease. Here, we will discuss different viral mechanisms for gene transduction and the different procedural techniques for administering treatment.
Most of the clinical trials involving gene therapy for IRDs are in Phase I or Phase II, with only a few trials making it to Phase III.1 Much of our understanding of the techniques and outcomes of viral vector delivery has come from clinical trials involving gene therapy for neovascular age-related macular degeneration and diabetic macular edema, in which gene vectors, such as RGX-314 (RegenxBio), are used to upregulate gene expression of an anti-VEGF molecule.
It started with voretigene neparvovec
Voretigene neparvovec aims to treat LCA caused by RPE65 gene mutations, which result in a deficiency of 11-cis-retinal. As a consequence, rod photoreceptors are unable to respond to light.2 Phase III results demonstrated functional visual improvements,3 and studies have demonstrated improved light sensitivity for up to three years, with subsequent decline.4,5
Subretinal delivery of the RPE65 gene has been well-tolerated with no adverse events related to the AAV vector.2,4,6 Humoral and cell-mediated responses to the AAV2 capsid and the RPE65 transgene were benign in all patients, with only one patient developing a cell-mediated response to AAV.4
Perifoveal chorioretinal atrophy, defined as progressively enlarging areas of chorioretinal atrophy not directly related to the touch-down site, has been reported in patients treated with voretigene neparvovec.7 These changes were more commonly seen within the area of the bleb, but were also observed outside of the bleb.8
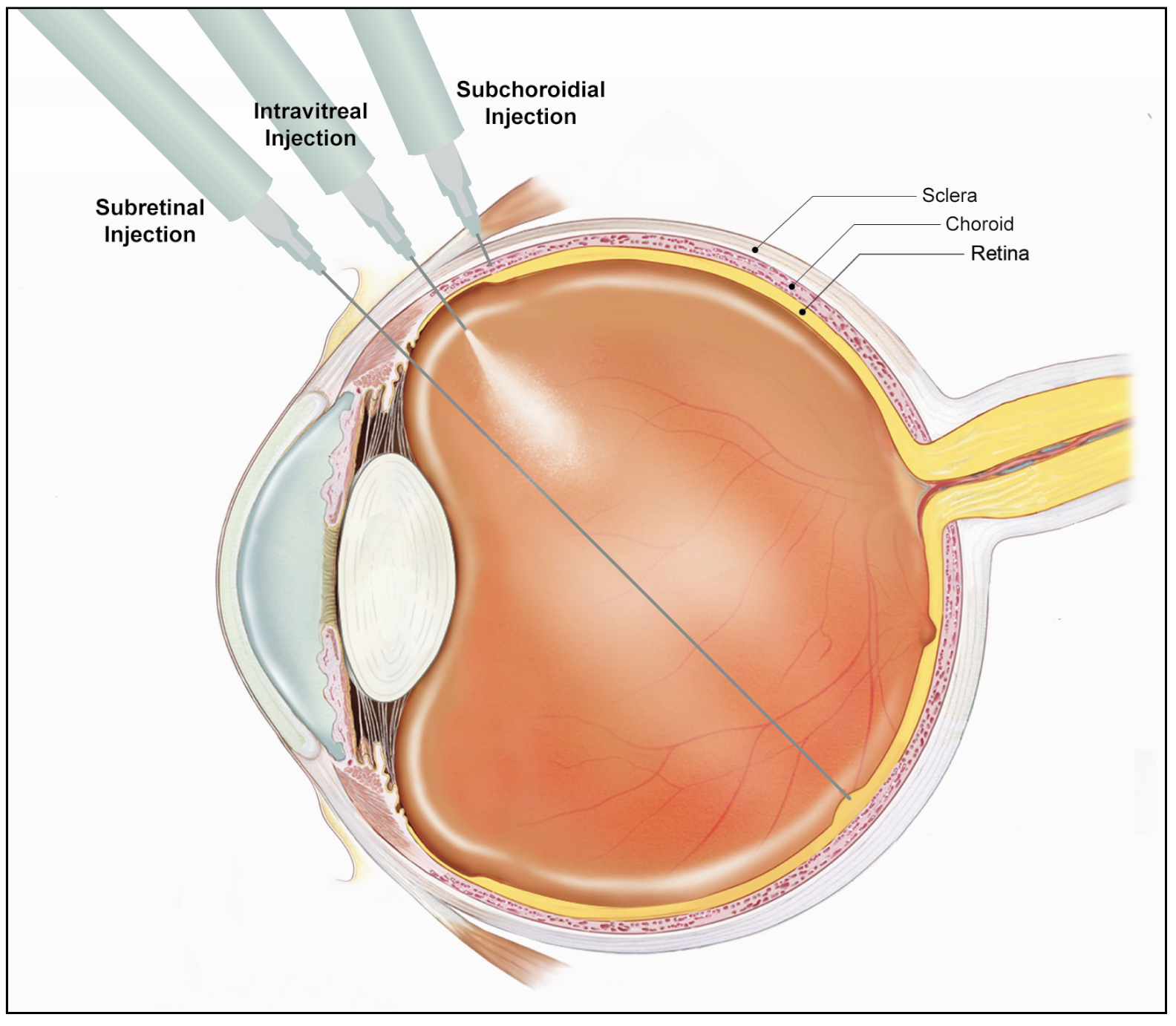 |
| The three modalities of gene therapy delivery. |
Viral vectors
Viral vectors for gene therapy include adenovirus, adeno-associated virus (AAV) and lentivirus. These vectors differ in their gene-carrying capacity, immunogenicity, cellular tropism and mutagenicity. AAV is the most widely used gene therapy vector.
AAV is a small, nonpathogenic virus that belongs to the Parvoviridae family. It contains a linear single-stranded DNA genome. AAV is advantageous in the use of gene delivery for many reasons, including:
- it transduces nondividing cells;
- it’s less immunogenic than other virus models;
- it doesn’t integrate into the host genome; and
- it maintains long-term gene expression.9
Because the eye is an immune-privileged site, only a limited immune reaction to the viral vector and transgene is observed.10 AAV is safe and has demonstrated long-term gene expression in the retina.2,4,6,11
Intravitreal administration
Most IRDs involve genetic mutation and dysfunction at the photoreceptors and/or retinal pigment epithelium levels. Compared with subretinal administration, intravitreal injections are less effective in delivering the gene vector to the outer retina.12 Intravitreal administration dilutes the vector in the vitreous cavity and it has to overcome a long diffusion distance to reach its target cells. Thus, higher vector concentrations are required.9,12
When administered intravitreally, the vector is exposed to the host’s immune system, resulting in neutralizing antibodies, intraocular inflammation, and reduced clinical efficacy.9 In animal models, subretinal administration of an AAV-RPE65 vector resulted in improved retinal function, while intravitreal administration did not.13
INFINITY, a Phase II trial of ADVM-022 (Ixo-vec, Adverum Biotechnologies), an AAV2 designed to deliver a transgene encoding aflibercept in patients with DME, was terminated and unmasked prematurely after a patient in the treatment arm experienced hypotony with panuveitis and vision loss.14,15 While similar devastating adverse events haven’t been reported in nAMD patients treated with ADVM-022, this adverse event has raised concern about the safety of intravitreal gene therapy.
Suprachoroidal gene therapy
The suprachoroidal space is a potential space between the inner sclera and the outer choroid. Catheters, hypodermic needles and microneedles can access this space.16,17 Microneedles have been developed to make suprachoroidal injection an office-based procedure done with topical anesthesia.
The Phase III PEACHTREE study demonstrated efficacy of suprachoroidal injections of triamcinolone acetonide for treatment of macular edema secondary to noninfectious uveitis.18 Subsequently, suprachoroidal injections have been explored as a potential treatment modality for gene therapy. Compared with subretinal injections where the vector remains in high concentration in the area of the bleb, suprachoroidal gene therapy has greater spread, allowing for expression through a larger area of RPE and outer retina.19
Suprachoroidal gene therapy is still in the early stages of development. Despite this, encouraging results have been reported with a well-tolerated safety profile of suprachoroidal RGX-314 in nAMD.20,21
Subretinal delivery
Subretinal gene therapy begins with a pars plana vitrectomy and inducing a posterior vitreous detachment if one isn’t already present. Triamcinolone particulate markers during this step may ensure complete hyaloid removal from the retinal surface. Hyaloidal remnants dramatically increase the overall challenge of bleb formation and agent delivery.
The vector is injected using a small-gauge cannula through the retina, creating a bleb between photoreceptors and RPE. A myriad of protocols and techniques exist for subretinal gene delivery. Many involve a pre-bleb with saline, others use the agent itself for bleb formation.
Bleb initiation may be challenging, depending on patient anatomy, patient movement and operator visualization. In many IRDs, bleb formation is a challenge because of the presence of subretinal fibrosis and excessive adhesion between the retina and RPE. Good candidates for subretinal gene therapy traditionally have clear lenses or have had cataract surgery performed by meticulous cataract surgeons, leaving minimal to no cortex. Exceeding 550 patients treated to date, the most commonly delivered subretinal gene therapy is RGX-314.
The advantage to this technique over intravitreal administration is that the subretinal space, because it is immune-privileged, has a lower likelihood of inflammation. Subretinal delivery of AAV results in gene transduction at the level of the photoreceptors, Muller cells and RPE cells.22
While, subretinal delivery of AAV is well tolerated, complications from the mode of administration have been reported. They include endophthalmitis, retinal detachment, macular hole and reduced visual acuity.2,4
Disadvantages of subretinal delivery
Disadvantages of this technique include iatrogenic separation of the retina from the RPE in bleb creation that results in photoreceptor compromise.13 This is especially costly in patients with IRDs, where photoreceptors are at baseline abnormal. As we mentioned, perifoveal chorioretinal atrophy has been seen in certain patients after administration of voretigene neparvovec. Pigment changes without atrophy have been observed following subretinal delivery of RGX-314 as well.14 Research into the causes is ongoing.
 |
Another disadvantage of subretinal delivery is that it requires surgery, often with general anesthesia in pediatric patients. Other drawbacks include the lack of predictability and reproducibility with subretinal bleb formation. During bleb formation, the viral vector may extend from the injection site symmetrically in a circle or asymmetrically in one direction.19 Viral vector may also escape into the vitreous cavity.19
Some immeasurable reflux out of the bleb will always occur. Blebs will also migrate before resorption, which can lead to unexpected effects such as macular transfection with superior retina bleb placement. These unpredictable surgical variables may result in variable amounts of transgene expression.19
Inflammation
Although the eye is immune-privileged, ocular inflammation almost always accompanies gene therapy regardless of the delivery mode delivery.23 Even when it’s not detected clinically, histologic evidence of inflammation at the cellular level is present. Inflammation is often responsive to immunosuppression, usually with local or systemic corticosteroids. Many variations of treatment protocols for inflammation have been reported, but no consensus exists on treatment strategies.23
Bottom line
Since the FDA approved voretigene neparvovec, research into gene therapies for the full spectrum of retinal diseases has expanded rapidly. Each of the three methods of administering retinal gene therapy have been evaluated, each with its own benefits and drawbacks. Inflammation is a constant concern with gene therapy administration, but the future is exciting for IRDs as well as more common conditions such as nAMD and DME. RS
REFERENCES
1. Nuzbrokh Y, Ragi SD, Tsang SH. Gene therapy for inherited retinal diseases. Ann Transl Med. 2021;9:1278.
2. Testa F, Maguire AM, Rossi S, et al. Three-year follow-up after unilateral subretinal delivery of adeno-associated virus in patients with Leber congenital Amaurosis type 2. Ophthalmology. 2013;120:1283-1291.
3. Maguire AM, Russell S, Wellman JA, et al. Efficacy, safety, and durability of voretigene neparvovec-rzyl in RPE65 mutation-associated inherited retinal dystrophy: Results of Phase 1 and 3 trials. Ophthalmology. 2019;126:1273-1285.
4. Bennett J, Wellman J, Marshall KA, et al. Safety and durability of effect of contralateral-eye administration of AAV2 gene therapy in patients with childhood-onset blindness caused by RPE65 mutations: a follow-on phase 1 trial. Lancet. 2016;388:661-672.
5. Jacobson SG, Cideciyan AV, Roman AJ, et al. Improvement and decline in vision with gene therapy in childhood blindness. N Engl J Med. 2015;372:1920-1926.
6. Russell S, Bennett J, Wellman JA, et al. Efficacy and safety of voretigene neparvovec (AAV2-hRPE65v2) in patients with RPE65-mediated inherited retinal dystrophy: A randomised, controlled, open-label, phase 3 trial. Lancet. 2017;390:849-860.
7. Gange WS, Sisk RA, Besirli CG, et al. Perifoveal chorioretinal atrophy after subretinal voretigene neparvovec-rzyl for RPE65-mediated Leber congenital amaurosis. Ophthalmol Retina. 2022;6:58-64.
8. Reichel FF, Seitz I, Wozar F, et al. Development of retinal atrophy after subretinal gene therapy with voretigene neparvovec. Br J Ophthalmol. Published onine May 24, 2022. Available at: https://bjo.bmj.com/content/early/2022/05/23/bjophthalmol-2021-321023.long
9. Ross M, Ofri R. The future of retinal gene therapy: evolving from subretinal to intravitreal vector delivery. Neural Regen Res. 2021;16:1751-1759.
10. Surace EM, Auricchio A. Versatility of AAV vectors for retinal gene transfer. Vision Res. 2008;48:353-359.
11. Day TP, Byrne LC, Schaffer DV, Flannery JG. Advances in AAV vector development for gene therapy in the retina. Adv Exp Med Biol. 2014;801:687-693.
12. Peng Y, Tang L, Zhou Y. Subretinal injection: A review on the novel route of therapeutic delivery for vitreoretinal diseases. Ophthalmic Res. 2017;58:217-226.
13. Acland GM, Aguirre GD, Bennett J, et al. Long-term restoration of rod and cone vision by single dose rAAV-mediated gene transfer to the retina in a canine model of childhood blindness. Mol Ther. 2005;12:1072-1082.
14. Khanani AM, Thomas MJ, Aziz AA, et al. Review of gene therapies for age-related macular degeneration. Eye (Lond). 2022;36:303-311.
15. Adverum provides update on ADVC-011 and the INFINITY trial in patients with diabetic macular edema [press release]. Redwood City, CA: Adverum Biotechnologies; July 22, 2021.
16. Kansara VS, Hancock SE, Muya LW, Ciulla TA. Suprachoroidal delivery enables targeting, localization and durability of small molecule suspensions. J Control Release. 2022;349:1045-1051.
17. Patel SR, Lin AS, Edelhauser HF, Prausnitz MR. Suprachoroidal drug delivery to the back of the eye using hollow microneedles. Pharm Res. 2011;28:166-176.
18. Yeh S, Khurana RN, Shah M, et al. Efficacy and safety of suprachoroidal CLS-TA for macular edema secondary to noninfectious uveitis: Phase 3 randomized trial. Ophthalmology. 2020;127:948-955.
19. Ding K, Shen J, Hafiz Z, et al. AAV8-vectored suprachoroidal gene transfer produces widespread ocular transgene expression. J Clin Invest. 2019;129:4901-4911.
20. Khanani AM. Suprachoroidal delivery of RGX-314 gene therapy for neovascular AMD: The Phase II AAVIATE study. Invest Ophthalmol Vis Sci. 2022;63:1497.
21. Vajzovic L. Suprachoroidal delivery of RGX-314 for diabetic retinopathy: The Phase II ALTITUDE study. Paper presented at the 55th annual Scientific Meeting of the Retina Society; Pasadena, CA; November 2, 2022.20.
22. Bennett J. Taking stock of retinal gene therapy: Looking back and moving forward. Mol Ther. 2017;25:1076-1094.
23. Chan YK, Dick AD, Hall SM, et al. Inflammation in viral vector-mediated ocular gene therapy: a review and report from a workshop hosted by the Foundation Fighting Blindness, 9/2020. Transl Vis Sci Technol. Published online April 1, 2021. Available at: https://tvst.arvojournals.org/article.aspx?articleid=2772451



