 |
 |
 |
As the last major ophthalmology conference of the year, the American Academy of Ophthalmology seems to host an inordinate share of pivotal clinical trial readouts in all fields of ophthalmology, but especially in retina. At the Retina 2019 Subspecialty Day, which actually takes up two full days, no fewer than 14 presentations were of late-breaking developments or first-time results of clinical trials. Here, we share summaries of five compelling study readouts from AAO.
First results of intravitreal gene therapy for nAMD
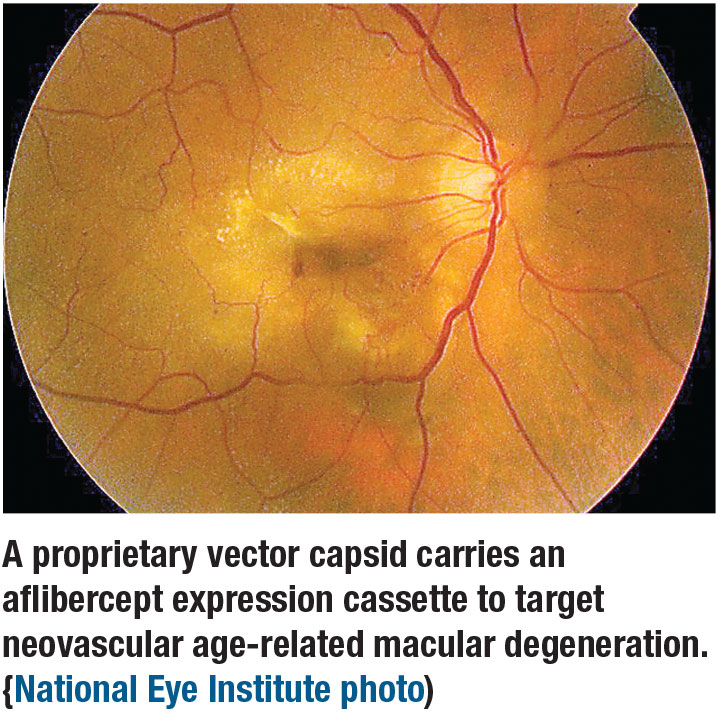
The Phase I OPTIC trial evaluated an intravitreal gene therapy, ADVM-022 (Adverum Biotechnologies), for the treatment of neovascular age-related macular degeneration. ADVM-022 uses a proprietary vector capsid, AAV.7m8, to carry an aflibercept expression cassette.1 It’s a one-time injection designed to increase transduction of retina cells and increase expression of the anti-VEGF protein.
Szilard Kiss, MD, of Weill Cornell Medical College in New York, reported on 24-week data from the first cohort dosed in OPTIC (n=6). Patients received an injection of aflibercept at baseline before the ADVM-022 injection. The cohort reported no serious adverse events.
Of the 19 adverse events potentially related to ADVM-022, 14 were mild in nature while five were moderate. No early clinically significant inflammation, vasculitis, retinitis or choroiditis occurred, and use of steroid drops did not worsen symptoms. Any anterior chamber cellular inflammation improved by week 24.
 |
The trial is enrolling three other cohorts, and 52-week data on the first cohort is due in the first half of 2020. The company is also preparing an investigational new drug application in diabetic retinopathy in the same time frame.
The take home: Average change in BCVA was a loss of 2 letters at 24 weeks, and central subfield thickness decreased 52.7 µm. No patients required a rescue injection over 24 weeks. Updated results out to 34 weeks reported no additional adverse events and an improvement in BCVA to a loss of 1.5 letters.
Dr. Kiss is a consultant and adviser to Adverum, RegenxBio and Fortress Bio, and holds equity in the companies. He is also a consultant and adviser to Genentech/Roche and Novartis.
First-time results of cell therapy for RP
A cell therapy treatment for retinitis pigmentosa is the focus of a Phase I/IIa trial that Pravin U. Dugel, MD, of Retinal Consultants of Arizona, Phoenix, reported on. The subretinal human retinal progenitor cell (hRPC) is the subject of a Phase I/IIa trial. The therapy uses cells isolated from fetal retinas. It can differentiate into retinal cells and can be cryopreserved with a shelf life of nine months. The therapy does not require immunosuppression.
Among the potential benefits of hRPC are the ability to deliver it directly into the subretinal space, on-demand shipment to the clinic and its ability to treat any genetic subtype of disease.
The Phase I trial treated 12 patients with doses of 250,000 or 500,000 fresh cells or 1 million cryopreserved cells. Because of the good safety profile, the Phase IIa trial consisted of 10 patients receiving a 1-million-cell dose.2
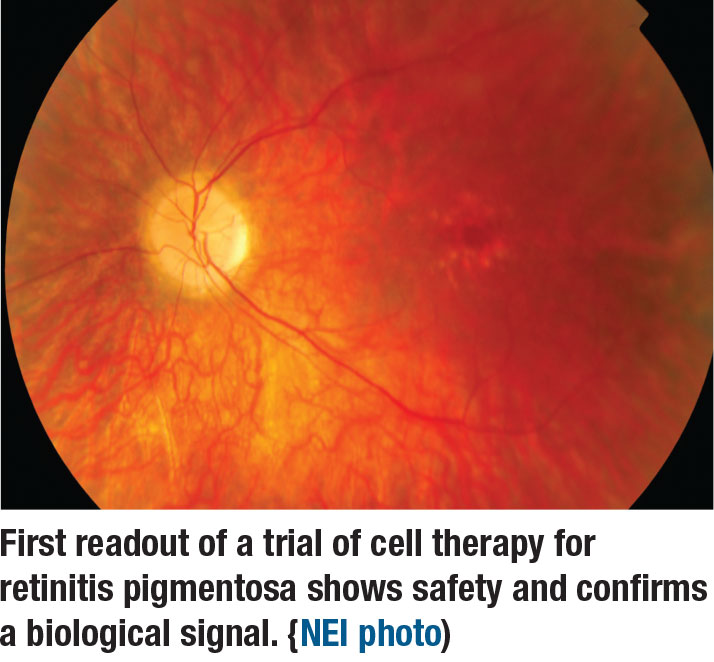 |
The first 22 patients tolerated dose escalation well and had no inflammation or proliferative vitreoretinopathy. There were two serious adverse ocular events unrelated to the drug. Two events leading to vision loss were related to the operation itself or patient selection: a retinal pigment epithelium tear; and persistent subretinal fluid. At 38 weeks post-treatment, BCVA improved an average of 12 letters in the treated eye vs. a 1-letter loss in the untreated eye.
The take home: The trial confirms a biological signal, although the speed of the effect varies among patients. The study results should help improve patient selection and surgical procedure standardization for future trials.
Dr. Dugel disclosed he is a consultant to ReNeuron PLC, the trial’s sponsor.
 |
Predictors of endophthalmitis and IVT anti-VEGF injections
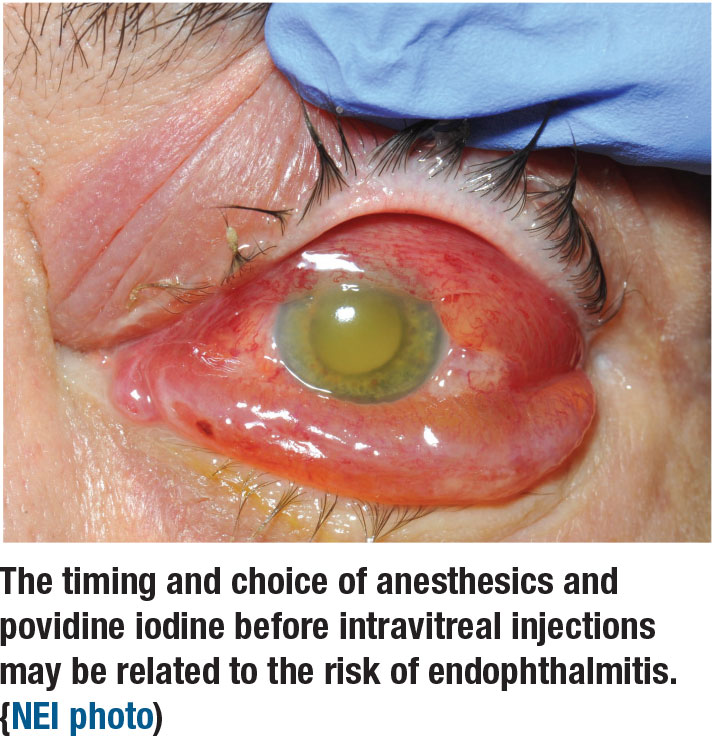
Complications of intravitreal anti-VEGF injections, while infrequent, are confounding and concerning for anyone who does a fair volume of procedures. Tarek Hassan, MD, reported on a retrospective analysis of 154,198 anti-VEGF injections by 15 retina specialists over three years at his practice, Associated Retinal Consultants in Royal Oak, Michigan.3 The goal was to identify predictive factors for endophthalmitis after IVT, evaluating each provider’s protocol. The preinjection protocol the providers followed consisted of povidone iodine (PVI) before and after anesthesia, typically subconjunctival or topical lidocaine.
Fifty-eight cases resulted in endophthalmitis, an incidence of 1:2,659, 41 percent culture positive. Multivariable analysis excluded same-day bilateral injections and cases by physicians with inconsistent injection protocols, resulting in 98,960 unilateral injections. Of those, 40 cases resulted in endophthalmitis, an incidence of 1:2,474, 42.5 percent culture positive.
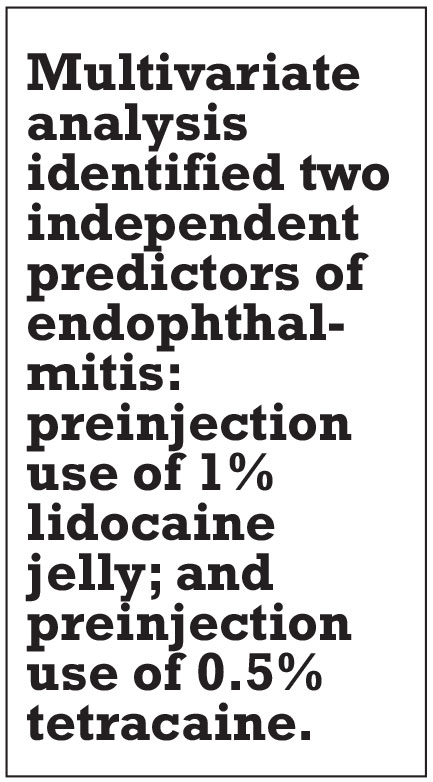
The take home: The multivariate analysis identified two independent predictors of endophthalmitis: preinjection use of 1% lidocaine jelly, with an 11 times greater odds of endophthalmitis (p<0.001); and preinjection use of 0.5% tetracaine with a fourfold risk (p=0.03). The study also noted that in vitro studies have shown an increased risk of microbial survival in the eye when lidocaine gel is used before the application of PVI,4,5 which may explain this study’s results. The analysis also determined that the use of 5% vs. 10% PVI tended to not improve or worsen endophthalmitis. Study results were published previously in Ophthalmology Retina.6
Dr. Hassan disclosed relationships with Alcon, Allergan, Genentech/Roche,
Novartis and Regeneron Pharmaceuticals.
 |
Microsecond pulsing laser vs. PDT in CSCR
A study involving patients with chronic central serous chorioretinopathy compared the effectiveness of microsecond pulsing (MSP) laser and photodynamic therapy, giving an edge to the former because of its less-invasive nature and improved patient comfort and cooperation.7
Jay Chhablani, MD, of the University of Pittsburgh reported on five studies comparing the two modalities, noting that outcomes were superior with MSP laser than half-fluence or half-dose PDT, but that the differences in improvements between the two groups were not significant. However, in one of the five surveyed studies, the study group reported a statistically significant visual acuity outcome with MSP laser.8
The take home: Choroidal neovascularization is a side effect of PDT while MSP laser didn’t show any side effects. Further, while the conventional MSP laser and navigated laser (Navalis) are “laser equivalent,” this study showed the navigated platform required significantly lower fluence with significantly fewer laser impacts than PDT, resulting in a higher rate of complete resolution with a statistically better best-corrected visual acuity. This effect may be attributed to the navigation. Additionally, the navigated platform can be used without a contact lens, “definitely improving the patient comfort and cooperation,” Dr. Chhablani said.
Dr. Chhablani has no disclosures.
 |
WF OCT-A surpasses FA for evaluating nonperfusion
Anti-VEGF therapy for diabetic macular edema has been known to improve the diabetic retinopathy severity scale (DRSS) score when evaluated with color retinal photography and stanch the progression of DR, but the role of anti-VEGF therapy in retinal perfusion, as imaged with fluorescein angiography, remains a matter of conjecture.
Investigators in France and Italy speculated that the variation in the ability to evaluate the role anti-VEGF has in peripheral retinal perfusion may be related to the inability to delineate areas of nonperfusion even with ultra-widefield FA. The researchers conducted a small study to evaluate the correlation between the number of DR lesions on ultra-widefield fundus photographs and nonproliferation on UWF FA.9
The study compared UWF color photography and UWF FA at baseline and one month after three monthly anti-VEGF injections in 18 eyes of 14 treatment-naïve patients. In results previously published in Retina, they reported no reperfusion of the arterioles or venules in or near the nonperfusion areas when the DRSS score improved at least one stage in 11 eyes (61 percent, p<0.0001).10 After three anti-VEGF injections, FA did not identify reperfusion of vessels despite DRSS improvement on color photographs.
Then the researchers used widefield optical coherence tomography angiography to evaluate DRSS, finding it improved at least one stage within three months after three monthly anti-VEGF injections or earlier in eight of 10 eyes, and that new vessels regressed. However, OCTA proved with better precision that no reperfusion occurred, even at the capillary level. These results are pending publication in Ophthalmology.11 All nonperfusion areas detected on UWF-FA were also detected on WF OCTA, and WF OCTA additionally detected some extra areas of nonperfusion that UWF-FA did not.
The take home: DRSS decorrelates from perfusion status after intravitreal anti-VEGF injections, and OCTA is superior to FA for evaluating nonperfusion. On UWF-FA, apparent changes in brightness of the background in areas of nonperfusion could lead to a misdiagnosis of reperfusion when WF OCTA finds no reperfusion. While WF OCTA can image an area larger than the seven standard field 30-degree color fundus photographs, UWF-FA may still be useful for areas WF OCTA can’t reach. RS
Presenter Ramin Tadayoni, MD, PhD, disclosed relationships with Alcon, Allergan, Carl Zeiss Meditec and Genentech/Roche.
REFERENCES
1. Kiss S. 24-week results of Phase 1 study of intravitreal gene therapy with ADVM-022 for neovascular AMD (OPTIC Trial). Presented at American Academy of Ophthalmology Subspecialty Day Retina 2019; October 11, 2019; San Francisco, CA.
2. Dugel P. Subretinal human retinal progenitor cells in retinitis pigmentosa: A Phase I/IIa study. Presented at AAO Subspecialty Day Retina 2019; October 12, 2019; San Francisco, CA.
3. Hassan T. Predictors of postinjection endophthalmitis: A multivariable analysis based on injection protocol and povidone iodine strength. Presented at AAO Subspecialty Day Retina 2019; October 11, 2019; San Francisco, CA.
4. Soshi RR, Leng T, Fung AE. Povidone-iodine before lidocaine gel anesthesia achieves surface antisepsis. Ophthalmic Surg Lasers Imaging. 2011;42:346-349.
5. Boden JH, Myers ML, Lee T, et al. Effect of lidocaine gel on povidone-iodine antisepsis and microbial survival. J Cataract Refract Surg. 2008;34:1773-1775.
6. Stem MS, Rao P, Lee AJ, et al. Predictors of endophthalmitis after intravitreal injection. Ophthalmol Retina. 2019;3:3-7.
7. Chhablani J. Comparison of microsecond pulsing laser versus photodynamic therapy in central serous chorioretinopathy. Presented at AAO 2019; October 15, 2019; San Francisco, CA.
8. Ntomoka CG, Rajesh B, Muriithi M, Goud A, Chhablanai J. Comparison of photodynamic therapy and navigated microsecond laser for chronic central serous chorioretinopathy. Eye. 2018;32:1079-1086.
9. Tadayoni R. Anti-VEGF treatment can diminish signs of diabetic retinopathy without reducing nonperfusion. Presented at AAO Subspecialty Day Retina 2019; October 12, 2019; San Francisco, CA.
10. Bonnin S, Dupas B, Lavia C, Erginay A, et al. Anti-vascular endothelial growth factor therapy can improve diabetic retinopathy score without change in retinal perfusion. Retina. 2019;39:426-434.
11. Courturier A, Rey PA, Erginay A, et al. Swept-source wide-field OCT-angiography versus ultra-wide-field fluorescein angiography assessments of retinal non-perfusion in diabetic retinopathy and edema treated with anti-VEGF. Ophthalmology. In press.



