Take-home points
|
 |
Bios Dr. Iyer is a vitreoretinal fellow at Tufts Medical Center/Ophthalmic Consultants of Boston. Dr. Shah is a retina specialist with Ophthalmic Consultants of Boston and fellowship co-director of the combined Tufts New England Medical Center/Ophthalmic Consultants of Boston vitreoretinal surgery fellowship. DISCLOSURES: Dr. Iyer has no relevant disclosures. Dr. Shah disclosed relationships with EyePoint Pharmaceuticals, Genentech/Roche and Regeneron Pharmaceuticals. |
It’s well known that exudation in neovascular age-related macular degeneration affects macular thickness and can influence best corrected visual acuity. Researchers have attempted to use central subfoveal thickness as a quantitative measurement from optical coherence tomography to predict visual outcomes in nAMD.
Absolute CST has been explored as a potential surrogate for total macular thickness, but it showed poor correlation with BCVA.1,2 More recently, several studies have looked at CST fluctuations and their impact on BCVA over time in patients treated with anti-VEGF injections in nAMD. Here, we review clinical trial data and real-world practices that have studied CST fluctuations and their correlation with BCVA.
Post-hoc analyses
= CATT and IVAN Trials. An analysis from the CATT (Comparison of Age-Related Macular Degeneration Treatment Trials) and IVAN (Inhibition of VEGF in Age-Related Macular Degeneration Treatments Trial) trials evaluated anatomic outcomes in anti-VEGF treated eyes.3-5
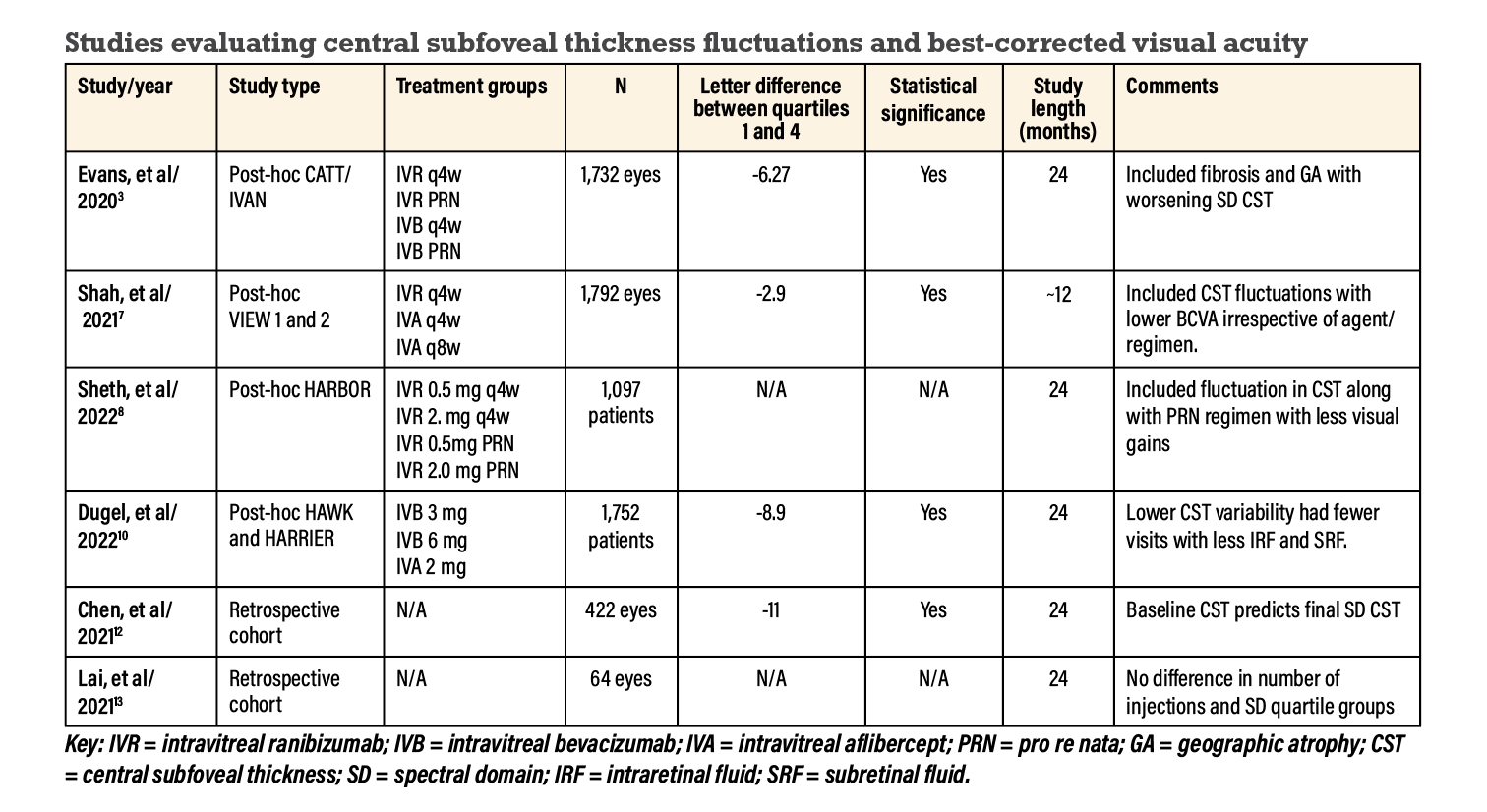
CATT and IVAN had four treatment groups: monthly and pro re nata with either bevacizumab or ranibizumab. This analysis investigated the visual and anatomic outcomes in 1,731 anti-VEGF-treated eyes for 24 months (Figure 1).3
The investigators’ measure of CST fluctuation was the standard deviation of the foveal center point thickness (FCPT) using time-domain or spectral domain OCT. They calculated FCPT and divided patients into quartiles, with higher quartiles exhibiting more fluctuation.
BCVA worsened significantly with increasing quartiles, with approximately 6 letters lost between the first and fourth quartile (-6.27 letters, confidence interval [CI] -8.45 to -4.09). No differences were observed in FCPT SD and BCVA between treatment regimens for both monthly and PRN treatments.
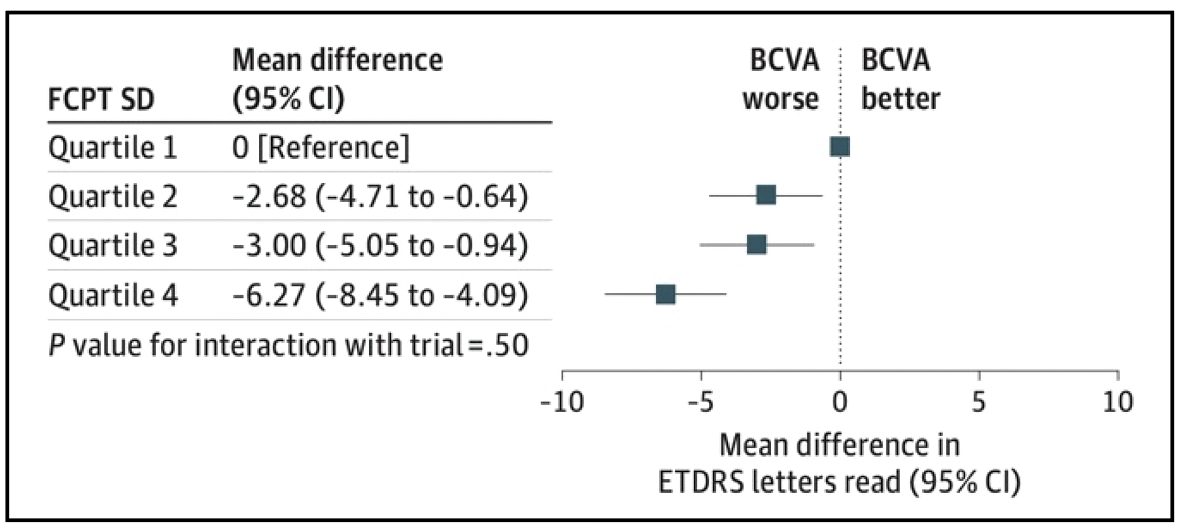 |
| Figure 1. The primary analysis model from the post-hoc study of the CATT and IVAN trials demonstrates estimates of associated foveal center-point thickness standard deviation by quartile with final best-corrected visual acuity.3 (Reproduced under Creative Commons License from Evans RN, Reeves BC, Maguire MG, et al. JAMA Ophthalmol. 2020;138:1043-1051.) |
 |
The analysis also observed increased fibrosis and geographic atrophy with increasing FCPT SD. The study concluded that increased variation in retinal thickness during treatment with anti-VEGF was associated with worse visual acuity, increased fibrosis and increased GA across different treatment regimens.3
= VIEW 1 and 2. A group from Johns Hopkins University in 2021 studied data from the VIEW 1 and 2 trials (VEGF Trap-Eye: Investigation of Efficacy and Safety in Wet AMD ).2,6 They allocated patients into three groups: ranibizumab 0.5 mg every 4 weeks (q4w); aflibercept 2 mg q4w; and aflibercept 2 mg q8w. At week 52, all groups converted to a PRN dosing for up to week 96.
This post-hoc analysis included 1,815 eyes analyzing the relationship between changes in CST (measured using time domain OCT) and BCVA. Linear regression analysis demonstrated weak correlations between changes in CST and BCVA with either agent or regimen at week 96 (p=0.69).2
We presented a similar post-hoc analysis of the VIEW 1 and 2 trials.7 Our unpublished data looked at CST fluctuations correlating with visual outcomes. We categorized CST fluctuations into quartiles and included 1,792 eyes from baseline to week 52. More eyes in the fourth quartile were treated with aflibercept q8w than with aflibercept or ranibizumab groups q4w. At week 52, mean visual gains were highest in the lower quartiles, with an approximate 3-letter loss between the first and fourth quartiles (-2.9 letters, CI -4.8 to -1.1, p=0.0017). The mean BCVA gains at week 52 were similar across all treatment groups and regimen.
= HARBOR. A post-hoc analysis from the Phase III HARBOR trial grouped patients with subfoveal nAMD into monthly or PRN ranibizumab 0.5 or 2 mg for 24 months.8,9 (HARBOR stands for Phase III, Double-masked, Multicenter, Randomized, Active Treatment-controlled Study of the Efficacy and Safety of 0.5 mg and 2.0 mg Ranibizumab Administered Monthly or on an As-needed Basis (PRN) in Patients with Subfoveal Neovascular Age-related Macular Degeneration.)
The analysis included 1,097 patients and evaluated CST variability over time with BCVA in response to ranibizumab. The researchers adopted fluctuation scores to assess CST variability, grouping them into quartiles from least to most fluctuation.
Patients in the lower-quartiles had greater visual gains at 24 months in both the monthly groups (approximately 10 letters on average) and PRN groups (approximately 9 letters). Quartile four had an 8-letter gain in the monthly regimen and 5-letter gain in the PRN group.
Thus, similar to the results from CATT, IVAN and VIEW 1 and 2, the HARBOR researchers observed less BCVA gain among eyes with greater CST fluctuation. They concluded that fluctuations in CST along with PRN dosing resulted in less visual gains than monthly treatment.8
= HAWK and HARRIER. Pravin Dugel, MD, and colleagues analyzed data from the HAWK and HARRIER trials to determine the association between CST and BCVA in patients treated with brolucizumab vs. aflibercept.10,11
From HAWK, the analysis included three groups randomized to brolucizumab 3 mg, brolucizumab 6 mg and aflibercept 2 mg. From HARRIER, patients were randomized to brolucizumab 6 mg and aflibercept 2mg. The aflibercept group received q8w treatment while the brolucizumab groups were injected q12w unless rescued sooner to q8w.
This analysis included 1,752 patients categorized into quartiles of increasing CST variability through CST SD values until week 96. The mean difference was 8.9 ETDRS letters from the first to the fourth quartile of CST SD (CI -10.6 to -7.3). Patients with lower CST variability also had fewer visits with the presence of intraretinal and subretinal fluid. The investigators concluded that CST variability may be a more reliable prognostic marker for visual outcomes than the presence of fluid alone.10
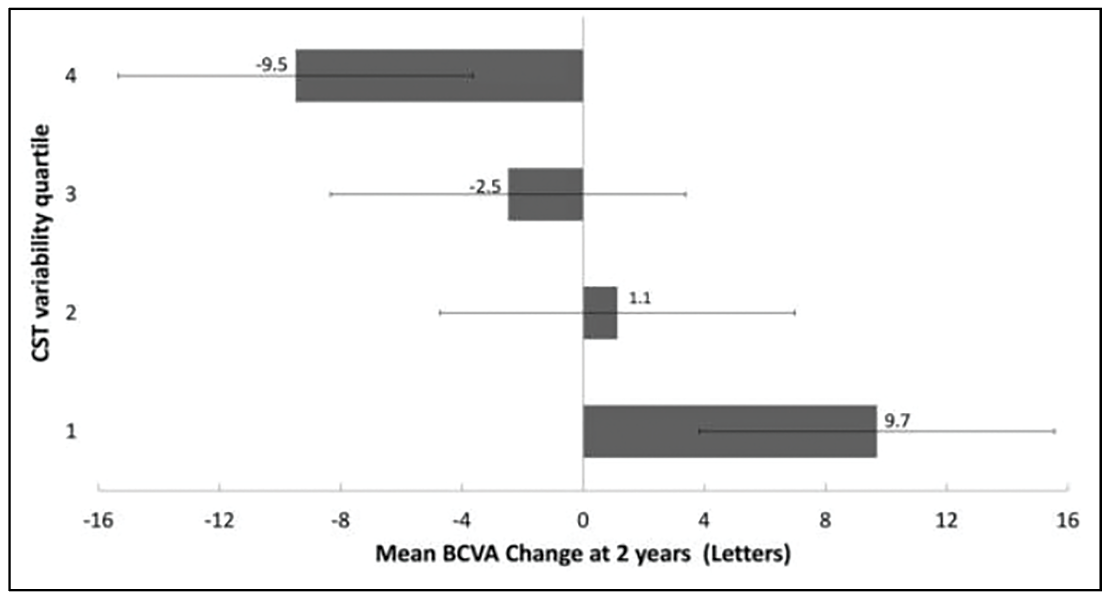 |
| Figure 2. A retrospective cohort real-world analysis documented mean best-corrected visual acuity change at two years for central subfoveal thickness variability among quartiles (error bars represent 95% confidence intervals). (Reproduced under Creative Commons License from Lai TY, Lai RY. J Personal Med. 2021;11:1024.) |
Real-world analyses
A retrospective cohort analysis of CST SD and BCVA over a 24-month period included 422 eyes without specificity of injection type or regimen.12 On average, the patients received 14 injections. This analysis divided CST SD into quartiles, and found it to be a negative predictor for change in BCVA (p< 0.001). There was also a difference of 11 letters between the first and fourth quartiles of CST SD (p<0.001). Baseline CST was a predictor of 24-month SD CST (p<0.001). The authors concluded that higher macular thickness fluctuations are related to poorer visual outcomes at 24 months in patients with nAMD treated with anti-VEGF injections.12
An earlier real-world retrospective study analyzed CST SD values and BCVA in patients receiving anti-VEGF agents for nAMD.13 It analyzed 64 over 24 months, again dividing the CST SD results into quartiles. The study found no significant difference in the number of injections in each group (p=0.21), but did find a significant correlation between increasing CST quartiles and worsening BCVA at two years (p< 0.0001) (Figure 2). The authors concluded that eyes undergoing anti-VEGF therapy with more stable CST variability were associated with better visual outcomes.13
 |
Bottom line
Clinical trial data and real-world experiences demonstrate that increasing CST fluctuations during treatment with anti-VEGF injections for nAMD is associated with poorer visual outcomes. Many
studies used a quartile system to categorize CST fluctuation, with a loss of approximately 3 to 11 letters when comparing lowest to highest CST fluctuation quartile.3,7,10,12
The particular anti-VEGF drug doesn’t seem to influence this relationship, but a PRN injection schedule along with higher CST fluctuation have worse visual outcomes, as the post-hoc HARBOR and VIEW 1 and 2 data demonstrated.7,10
CST fluctuation was also associated with increased fibrosis, GA and more macular fluid, leading to worse visual outcomes.3,10 Disease characteristics, such as the size, location and subtype of the choroidal neovascular membrane, may influence CST fluctuation.
CST fluctuation is a reliable prognostic marker in nAMD that may have utility in future clinical trials. Further, this finding supports the potential value of maintaining stable CST, presumably with less leakage, to optimize visual gains. RS
REFERENCES
1. Simader C, Ritter M, Bolz M, et al. Morphologic parameters relevant for visual outcome during anti-angiogenic therapy of neovascular age-related macular degeneration. Ophthalmology. 2014;121:1237-1245.
2. Nanegrungsunk O, Gu SZ, Bressler SB, et al. Correlation of change in central subfield thickness and change in visual acuity in neovascular AMD: Post hoc analysis of VIEW 1 and 2. Am J Ophthalmol. 2022;238:97-102.
3. Evans RN, Reeves BC, Maguire MG, et al. Associations of variation in retinal thickness with visual acuity and anatomic outcomes in eyes with neovascular age-related macular degeneration lesions treated with anti–vascular endothelial growth factor agents. JAMA Ophthalmol. 2020;138:1043-1051.
4. Martin DF, Maguire MG, Ying Ga, Grunwald JE, Fine SL, Jaffe GJ. Ranibizumab and bevacizumab for neovascular age-related macular degeneration. New Engl J Med. 2011;364:1897-1908.
5. Chakravarthy U, Harding SP, Rogers CA, et al. Alternative treatments to inhibit VEGF in age-related choroidal neovascularisation: 2-year findings of the IVAN randomised controlled trial. The Lancet. 2013;382:1258-1267.
6. Heier J. Intravitreal aflibercept (VEGF trap-eye) in wet age-related macular degeneration. Ophthalmology. 2012;119:3537-3548.
7. Shah CP, et al. Visual impact of central subfield thickness (CST) fluctuation in patients with nAMD treated with anti-VEGFs in VIEW trials. Unpublished data presented at The Retina Society. Sept 30, 2021. Chicago, Illinois, USA.
8. Sheth V, D'Rozario M, Gune S, Blotner S. Fluctuations in central foveal thickness and association with vision outcomes with anti-VEGF therapy for nAMD: HARBOR post hoc analysis. BMJ Open Ophthalmol. 2022;7:e000957.
9. Sadda SR, Tuomi LL, Ding B, Fung AE, Hopkins JJ. Macular atrophy in the HARBOR study for neovascular age-related macular degeneration. Ophthalmology. 2018;125:878-886.
10. Dugel PU, Jhaveri CD, Chakravarthy U, et al. Effect of retinal thickness variability on visual outcomes and fluid persistence in neovascular age-related macular degeneration: A post hoc analysis of the HAWK and HARRIER studies. Retina. 2022;42:511-518.
11. Dugel PU, Singh RP, Koh A, et al. HAWK and HARRIER: Ninety-six-week outcomes from the Phase 3 trials of brolucizumab for neovascular age-related macular degeneration. Ophthalmology. 2021;128:89-99.
12. Chen ER, Chen AX, Greenlee TE, et al. Macular thickness fluctuation in neovascular age-related macular degeneration treated with anti-vascular endothelial growth factor. Can J Ophthalmol. 2022;57:350-356.
13. Lai TY, Lai RY. Association between retinal thickness variability and visual acuity outcome during maintenance therapy using intravitreal anti-vascular endothelial growth factor agents for neovascular age-related macular degeneration. J Personal Med. 2021;11:1024.



