ABOUT THE AUTHORS | |
 | Dr. Scott is professor of ophthalmology and public health sciences, Penn State Hershey Eye Center, Penn State College of Medicine, Hershey, Pa. She has no disclosures. |
 | Dr. Flynn is the J. Donald M. Gass Distinguished Chair and professor of ophthalmology at Bascom Palmer Eye Institute, University of Miami Miller School of Medicine. He has no disclosures to report. |
Quantifying Risk of Endophthalmitis
With the widespread use of the intravitreal injection technique has come an increased concern regarding the risk of post-injection endophthalmitis. Because most patients treated with anti-VEGF agents receive a series of injections over months or years, it is important to distinguish between per-injection rates of endophthalmitis versus per-patient (or cumulative) rates of endophthalmitis over the course of treatment. While reported per-injection rates of endophthalmitis are generally very low in series of patients treated with anti-VEGF agents, per-patient rates may be close to 1 percent over a two-year course of therapy.Recent series from Florida (20 cases of endophthalmitis out of 121,285 intravitreal injections, or a rate of 0.016 percent),3 Denmark (two cases out of 7,584 injections, or a rate of 0.026 percent),4 Australia (two cases out of 9.162 injections, 0.022 percent)5 and Massachusetts (three cases out of 10,208 injections, 0.029 percent)6 indicate an endophthalmitis rate of 1 per 3,000 intravitreal anti-VEGF injections or less. A population-based study in the United Kingdom estimated the per-injection rate of endophthalmitis following intravitreal anti-VEGF injection to be 0.025 percent.7 A review of the United States Medicare claims database revealed a per-injection rate of 0.09 percent for endophthalmitis associated with intravitreal anti-VEGF injections administered to patients with age-related macular degeneration (AMD).8
In the Comparison of Age-related Macular Degeneration Treatments Trials (CATT), a prospective, multicenter, randomized clinical trial comparing intravitreal ranibizumab (Lucentis, Genentech) and intravitreal bevacizumab (Avastin, Genentech) in 1,107 patients with neovascular AMD, the per-patient rate of endophthalmitis over two years was 0.7 percent with intravitreal ranibizumab and 1.2 percent with intravitreal bevacizumab (p=0.38).9 A meta-analysis of 43 studies reported that endophthalmitis occurred after 197 of 350,535 intravitreal anti-VEGF injections, a rate of 0.056 percent.10
While most postsurgical endophthalmitis cases are believed to be related to the patient’s ocular surface flora, with the most common causative organisms being coagulase-negative Staphylococcus species,11-14 many cases of endophthalmitis associated with intravitreal injection may be related to droplet transmission from the patient or from the health care providers involved with the intravitreal injection.
Table 1. Guideline Areas with General Agreement Among Committee Members24 |
| • Povidone-iodine (5-10 percent) should be the last agent applied to the intended injection site before injection. If a gel anesthetic is used, povidone-iodine should be applied both before and after gel application, because retained gel may prevent povidone-iodine from contacting the conjunctival surface of the injection site. • Topical antibiotics pre-, peri- or postinjection are unnecessary. • No evidence supports the routine use of a sterile drape. • Avoid contamination of the needle and injection site by the eyelashes or the eyelid margins. • Avoid extensive massage of the eyelids either pre- or postinjection (to avoid meibomian gland expression). • Use adequate anesthetic for a given patient (topical drops, gel and/or subconjunctival injection). • Use sterile or nonsterile gloves as consistent with modern office practice, combined with strong agreement regarding the need for hand washing before and after patient contact. • Either surgical masks should be used or both the patient and providers should minimize speaking during the injection preparation and procedure to limit aerosolized droplets containing oral contaminants from the patient and/or provider. • Monitor IOP both pre- and post-injection. • Routine anterior chamber paracentesis is not recommended. |
| Adapted from Avery RL, Bakri SJ, Blumenkranz MS, et al. Intravitreal injection technique and monitoring: updated guidelines of an expert panel. Retina (suppl). 2014;34:S1-S188 |
Colin McCannel, MD, at Jules Stein Eye Institute at UCLA, summarized 54 cases of endophthalmitis following 105,532 intravitreal injections of anti-VEGF agents, and found that, of the culture-positive cases, Staphylococcus (n=17, 65 percent) and Streptococcus (n=8, 31 percent) were the most common causative organisms.22
In contrast, in case series of postsurgical endophthalmitis, the proportion of culture-positive cases attributed to Streptococcus species ranged from 0 to 9 percent.22
Similarly, Ninel Z. Gregori, MD, and colleagues at Bascom Palmer Eye Institute reported on a series of 20 patients with endophthalmitis after 121,285 intravitreal injections of anti-VEGF agents.3 Of the nine (45 percent) culture-positive cases, five (56 percent) were Streptococcus species, three (33 percent) were coagulase-negative Staphylococcus and one (11 percent) was Bacillus non-anthracis
.
John B. Fileta, MD, and colleagues at Penn State College of Medicine reported a meta-analysis which included 197 cases of endophthalmitis out of 350,535 intravitreal anti-VEGF injections.10 The most commonly isolated organisms were coagulase-negative Staphylococcus (38 percent) and Streptococcus (29 percent). An analysis of conjunctival flora in patients undergoing intravitreal injections identified Streptococcus species in only three of 71 cultured isolates (4.2 percent), supporting the hypothesis that such organisms come from respiratory droplets instead of the patient’s conjunctival flora.23
Strategies to Reduce Risks
Strategies that may be important in reducing the risk of complications associated with intravitreal injections include attention to issues before, during and after the injection. Tables 1 and 2 summarize intravitreal injection procedure guidelines developed as a result of expert committee deliberations conducted after a review of published and unpublished studies and case series. Table 3 provides an appropriate sequence of events for the administration of intravitreal injection.24An active external infection, including significant blepharitis, should be treated prior to injection. In addition, eyelid abnormalities such as ectropion have been reported as risk factors for endophthalmitis. Ocular surface bacteria represent an important source of bacteria-causing postoperative endophthalmitis11-14 and postintravitreal injection endophthalmitis.10,22
Thus, one strategy to reduce the risk of endophthalmitis is to reduce or eliminate the bacteria on the patient’s ocular surface and eyelids. While this may be achieved in various ways (povidone-iodine, topical antibiotics, eyelid hygiene and sterile isolation of the surgical site), povidone-iodine (Figure 2) is the only agent that has been demonstrated to reduce the risk of postoperative endophthalmitis in a prospective study of cataract surgery.25
Lid scrubs have been associated with a significant increase in bacterial flora, so avoid excessive eyelid manipulation prior to injection. Since true contact allergy to povidone-iodine is rare, and anaphylaxis after ophthalmic application of povidone-iodine has not been reported, a reported history of contact allergy to povidone-iodine can be verified with a skin patch test. Conjunctival exposure to 5 percent povidone-iodine for a period of 30 seconds achieves a significant reduction in the bacterial colony-forming units and appears to be an adequate contact time before intravitreal injection.26
Antibiotics: No Proven Effect
Topical antibiotics have been demonstrated to reduce ocular surface bacteria, but have not been proven to reduce the risk of endophthalmitis.27-30 Despite evidence that topical antibiotics reduce the conjunctival bacterial load,29,31,32 several studies have demonstrated that the addition of preinjection or postinjection topical antibiotics to povidone-iodine antisepsis does not decrease the rates of endophtalmitis compared with the use of povidone-iodine alone.33-35Table 2. Guideline Areas With No Clear Consensus Among Committee Members24 |
| • Need for povidone–iodine application to the eyelids, including the eyelashes and eyelid margins. All agreed that when povidone-iodine is applied to the eyelashes and eyelid margins, eyelid scrubbing or eyelid pressure adequate to express material from the meibomian gland should be avoided. • Use of a speculum. (Some prevent contact between the needle/injection site and the eyelashes and eyelids with manual lid retraction.) • Need for pupillary dilation and postinjection dilated examination of the posterior segment. (Although some viewed the return of formed vision as sufficient, others routinely dilate the pupil and examine the posterior segment after injection.) • Use of povidone-iodine flush. (Most preferred drops only and saw no benefit to allowing the povidone-iodine to dry before injection.) |
| Adapted from Avery RL, Bakri SJ, Blumenkranz MS, et al. Intravitreal injection technique and monitoring: updated guidelines of an expert panel. Retina (suppl). 2014;34:S1-S18 |
Moreover, use of topical antibiotics has been associated with increased bacterial resistance rates of ocular surface flora in patients undergoing intravitreal injections.37-39 In one prospective study over a one-year period, coagulase-negative Staphylococcus resistance to gatifloxacin and moxifloxacin roughly doubled, from rates of 39 percent and 34 percent, respectively, prior to intravitreal injections, to 67 percent and 70 percent, respectively, following treatment.37,38 These eyes were initially treatment-naïve, and all received four monthly intravitreal injections, followed by intravitreal injections as needed.
After each injection, patients were administered one drop of their assigned fluoroquinolone to the injected eye and were instructed to instill one drop of the fluoroquinolone q.i.d. for four days. Over the same one-year study period, untreated fellow eyes of the same patients did not develop an increase in fluoroquinolone-resistant coagulase-negative Staphylococcus strains. Thus, due to emerging antimicrobial resistance and lack of evidence supporting a reduction in endophthalmitis rates, topical antibiotic use for prophylaxis of endophthalmitis following intravitreal injection is not the current standard of care.
Other Preventative Measures
Because of the evidence that the underlying causative mechanism for some cases of endophthalmitis after intravitreal injection might be related to respiratory droplet transmission from the patient or the health-care providers involved with the injection, an expert panel recommended the patient and providers either wear surgical masks or minimize speaking during injection preparation and the procedure.24,40Another strategy involves the use of a sterile lid speculum during the injection procedure to avoid needle contact with lids and lashes. However, the most recent “guidelines” paper listed the use of a lid speculum as having “no consensus” among the panel members because many no longer used a speculum.24 The use of a sterile drape is also optional, but gloves, part of universal precautions, are appropriate. However, some panelists on the expert committee prefer to not use gloves.
The first step involves administration of a sterile topical anesthetic. Retina specialists may consider subconjunctival anesthesia, but this requires additional instrumentation and manipulation that may be associated with increased surface flora. If subconjunctival anesthesia is used, keep in mind that the needle used for intravitreal injection passes through the subconjunctival space filled with anesthetic and that surface bacteria may have been introduced beneath the conjunctiva.
Although lidocaine gel has been known to improve patient comfort during intravitreal injections, it may also serve as a barrier, reducing the ability of povidone-iodine to contact the ocular surface and reduce the risk of endophthalmitis. Even if povidone-iodine is administered prior to lidocaine gel (in an attempt to bypass the potential barrier effect), one should recognize that the commercially available lidocaine gel is not prepared as a sterile formulation and, therefore, the injection needle may become contaminated as it passes through the lidocaine gel and before it enters the intravitreal cavity. Thus, if a gel anesthetic is used, povidone-iodine should be applied both before and after the gel. Care should be taken to avoid pressure to the eyelids, eyelid margins and the adnexa due to the potential for release of resident bacteria.
Table 3. Intravitreal Injection Procedure: An Appropriate Sequence of Events | |||||
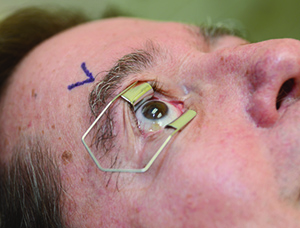 | 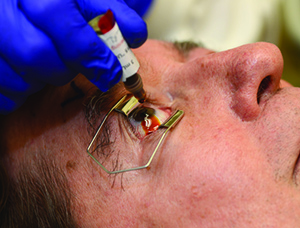 | 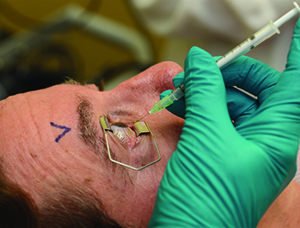 | |||
| 1. Either surgical masks should be used or both the patient and providers should minimize speaking during the injection preparation and procedure. 2. Take a procedural time-out to verify patient, agent and laterality. 3. Apply liquid anesthetic drops to the ocular surface. 4. Apply povidone-iodine to the eyelashes and eyelid margins. (This is optional; most use 10% concentration.) 5. Retract the eyelids away from the intended injection site for the duration of the procedure. | 6. Apply povidone-iodine (most use 5%) to the conjunctival surface, including the intended injection site, at least 30 seconds before injection. 7. If additional anesthetic is applied, reapply povidone-iodine to the intended injection site immediately before injection (again, most use 5%). 8. Insert the needle perpendicular to the sclera, 3.5 to 4 mm posterior to the limbus (3 to 5 mm in pseudophakic or aphakic eyes) between the vertical and horizontal rectus muscles. | ||||
Approach with the Needle
Intravitreal injections should be given between the horizontal and vertical rectus muscles at the pars plana (Figure 3), 3.5 to 4 mm posterior to the limbus in phakic eyes and 3 to 3.5 mm posterior to the limbus in pseudophakic or aphakic eyes.While the inferotemporal quadrant is generally the preferred site of injection due to such factors as ease of exposure (no need to pass the needle over the bridge of the nose or the brow), patient-specific considerations and the injecting physician’s preference should dictate quadrant selection.
Although oblique and tunneled needle insertions have been described as attempts to minimize drug reflux after injection, a perpendicular injection approach is convenient and preferred in most settings.24
Needle gauge is selected based on the drug being injected. A 30-gauge or smaller needle is generally preferred for nonviscous drugs. Larger gauge needles may be considered for suspensions and for more viscous solutions. Needle length should be 5/8 inch (18 mm) or shorter, but long enough to permit complete penetration of the pars plana.24
The intraocular pressure (IOP) should be monitored postinjection and therapy for elevated IOP administered as indicated. Before the patient leaves the office, the injecting physician should assess IOP or confirm the presence of formed vision and give the patient 24-hour emergency contact information.
Patients and/or caregivers also need to be educated about the importance of avoiding eye rubbing and how to recognize and report immediately the signs and symptoms of endophthalmitis, retinal detachment and intraocular hemorrhage.
Conclusion
To optimize the outcomes associated with intravitreal injection, the retina specialist and staff should pay careful attention to reducing the risk of complications. Treatment outcomes depend not only on the safety and efficacy of the pharmacotherapy being delivered, but also on the safety and potential adverse events associated with the procedure itself. RSReferences
1. Ramulu PY, Do DV, Corcoran KJ, Corcoran SL, Robin AL. Use of retinal procedures in Medicare beneficiaries from 1997 to 2007. Arch Ophthalmol. 2010;128:1335-1340.2. Williams GA. Intravitreal injections: health policy implications. Rev Ophthalmol 2014;21:62-64.
3. Gregori NZ, Flynn HW Jr, Schwartz SG, et al. Current infectious endophthalmitis rates after intravitreal injections of anti-vascular endothelial growth factor agents and outcomes of treatment. In press in OSLI Retina; 2015.
4. Rasmussen A, Bloch SB, Fuchs J, et al. A 4-year longitudinal study of 555 patients treated with ranibizumab for neovascular age-related macular degeneration. Ophthalmology. 2013;120:2630-2636.
5. Gillies MC, Walton R, Simpson JM, et al. Prospective audit of exudative age-related macular degeneration: 12-month outcomes in treatment-naïve eyes. Invest Ophthalmol Vis Sci. 2013;54:5754-5760.
6. Englander M, Chen TC, Paschalis EI, Miller JW, Kim IK. Intravitreal injections at the Massachusetts Eye and Ear Infirmary: analysis of treatment indications and postinjection endophthalmitis rates. Br J Ophthalmol. 2013;97:460-465.
7. Lyall DA, Tey A, Foot B, et al. Post-intravitreal anti-VEGF endophthalmitis in the United Kingdom: incidence, features, risk factors, and outcomes. Eye (Lond). 2012;26:1517-1526.
8. Day S, Acquah K, Mruthyunjaya P, et al. Ocular complications after anti-vascular endothelial growth factor therapy in Medicare patients with age-related macular degeneration. Am J Ophthalmol. 2011;152:266-272.
9. Comparison of Age-related Macular degeneration Treatment Trials (CATT) Research Group, Martin DF, Maguire MG, et al. Ranibizumab and bevacizumab for treatment of neovascular age-related macular degeneration: 2-year results. Ophthalmology. 2012:119:1388-1398.
10. Fileta JB, Scott IU, Flynn HW Jr. Meta-analysis of infectious endophthalmitis after intravitreal injection of anti-vascular endothelial growth factor agents. Ophthalmic Surg Lasers Imag Retina. 2014;45:143-149.
11. Speaker MG, Milch FA, Shah MK, et al. Role of external bacterial flora in the pathogenesis of acute postoperative endophthalmitis. Ophthalmology. 1991;98:639-649; discussion 650.
12. Bannerman TL, Rhoden DL, McAllister SK, et al. The source of coagulase-negative staphylococci in the Endophthalmitis Vitrectomy Study. A comparison of eyelid and intraocular isolates using pulsed-field gel electrophoresis. Arch Ophthalmol. 1997;115:357-361.
13. Han DP, Wisniewski SR, Wilson LA, et al. Spectrum and susceptibilities of microbiologic isolates in the Endophthalmitis Vitrectomy Study. Am J Ophthalmol. 1996;122:1-17.
14. Leong JK, Shah R, McCluskey PJ, et al. Bacterial contamination of the anterior chamber during phacoemulsification cataract surgery. J Cataract Refract Surg. 2002;28:826-833.
15. Gordon DF, Jong BB. Indigenous flora from human saliva. Appl Microbiol. 1968;16:428-429.
16. McCarthy C, Snyder Ml, Parker RB. The indigenous oral flora of man. I. The newborn to the 1-year-old infant. Arch Oral Biol. 1965;10:61-70.
17. Veringa E, van Belkum A, Schellekens H. Iatrogenic meningitis by Streptococcus salivarius following lumbar puncture. J Hosp Infect. 1995;29:316-318.
18. Sheretz RJ, Reagan DR, Hampton KD, et al. A cloud adult: the Staphylococcus aureus-virus interaction revisited. Ann Intern Med. 1996;124:539-547.
19. O’Kelly SW, Marsh D. Face masks and spinal anaesthesia. Br J Anaesth. 1993;70:239.
20. McLure HA, Talboys CA, Yentis SM, Azadian BS. Surgical face masks and downward dispersal of bacteria. Anaesthesia. 1998;53:624-626.
21. Trautmann M, Lepper PM, Schmitz FJ. Three cases of bacterial meningitis after spinal and epidural anesthesia. Eur J Clin Micobiol Infect Dis. 2002;21:43-45.
22. McCannel CA. Meta-analysis of endophthalmitis after intravitreal injection of anti-vascular endothelial growth factor agents. Causative organisms and possible prevention strategies. Retina. 2011;31:654-661.
23. Moss JM, Sanislo SR, Ta CN. Antibiotic susceptibility patterns of ocular bacterial flora in patients undergoing intravitreal injections. Ophthalmology. 2010;117:2141-2145.
24. Avery RL, Bakri SJ, Blumenkranz MS, et al. Intravitreal injection technique and monitoring: updated guidelines of an expert panel. Retina (suppl). 2014;34:S1-S18
25. Speaker MG, Menikoff JA. Prophylaxis of endophthalmitis with topical povidone-iodine. Ophthalmology. 1991;98:1769-1775.
26. Friedman DA, Mason JO, Emond T, McGwin G. Povidone-iodine contact time and lid speculum use during intravitreal injection. Retina. 2013;33:975-981.
27. Isenberg S, Apt L, Yoshimori R, Khwarg S. Chemical preparation of the eye in ophthalmic surgery IV: comparison of povidone-iodine on the conjunctiva with a prophylactic antibiotic. Arch Ophthalmol. 1985;103:1340-1342.
28. Grimes S, Mein C, Trevino S. Preoperative antibiotic and povidone-iodine preparation of the eye. Ann Ophthalmol. 1991;23:263-266.
29. Ta CN, Egbert PR, Singh K, et al. Prospective randomized comparison of 3-day versus 1-hour preoperative ofloxacin prophyl-axis for cataract surgery. Ophthalmology. 2002;109:2036-2041.
30. Osher RH, Amdahl LD, Cheetham JK. Antimicrobial efficacy and aqueous humor concentration of preoperative and postoperative topical trimethoprim/polymyxin B sulfate versus tobramycin. J Cataract Refract Surg. 1994;20:3-8.
31. Vasavada AR, Gajjar D, Raj SM, Vasavada V, Vasavada V. Comparison of 2 moxifloxacin regimens for preoperative prophylaxis: prospective randomized triple-masked trial. Part 2: residual conjunctival flora. J Cataract Refract Surg. 2008;34:1383-1388.
32. Moss JM, Nguyen D, Liu YI, et al. Comparison of one-day versus one-hour application of topical gatifloxacin in eliminating conjunctival bacterial flora. Ophthalmology. 2008:215:2013-2016.
33. Bhatt SS, Stepien KE, Joshi K. Prophylactic antibiotic use after intravitreal injection: effect on endophthalmitis rate. Retina. 2011;31:2032-2036.
34. Moss JM, Sanislo SR, Ta CN. A prospective randomized evaluation of topical gatifloxacin on conjunctival flora in patients undergoing intravitreal injections. Ophthalmology. 2009;116:1498-1501.
35. Cheung CS, Wong AW, Lui A, et al. Incidence of endophthalmitis and use of antibiotic prophylaxis after intravitreal injections. Ophthalmology. 2012;119:1609-1614.
36. Halachmi-Eyal O, Lang Y, Keness Y, Miron D. Preoperative topical moxifloxacin 0.5 percent and povidone-iodine 5.0 percent versus povidone-iodine 5.0 percent alone to reduce bacterial colonization in the conjunctival sac [published correction appears in J Cataract Refract Surg. 2010;36:535]. J Cataract Refract Surg. 2009;35:2109-2114.
37. Kim SJ, Toma HS. Ophthalmic antibiotics and antimicrobial resistance: a randomized, controlled study of patients undergoing intravitreal injections. Ophthalmology. 2011;118:1358-1363.
38. Kim SJ, Toma HS. Antimicrobal resistance and ophthalmic antibiotics: 1-year results of a longitudinal controlled study of patients undergoing intravitreal injections. Arch Ophthalmol. 2011;129:1180-1188.
39. Milder E, Vander J, Shah C, Garg S. Changes in antibiotic resistance patterns of conjunctival flora due to repeated use of topical antibiotics after intravitreal injection. Ophthalmology. 2012;119:1420-1424.
40. Schimel AM, Scott IU, Flynn HW Jr. Endophthalmitis after intravitreal injections: should the use of face masks be the standard of care? Arch Ophthalmol. 2011;129:1607-1609.



