 |
The first gene therapies for retinal disease targeted inherited genetic disorders, such as Spark Therapeutics’ Luxturna for treatment of biallelic RPE65 mutation-associated retinal dystrophy, the first approved gene therapy for inherited disease. However, increasingly early phase gene therapy programs are targeting age-related macular degeneration, both the wet and dry forms, although inherited retinal diseases still remain the most desired target.
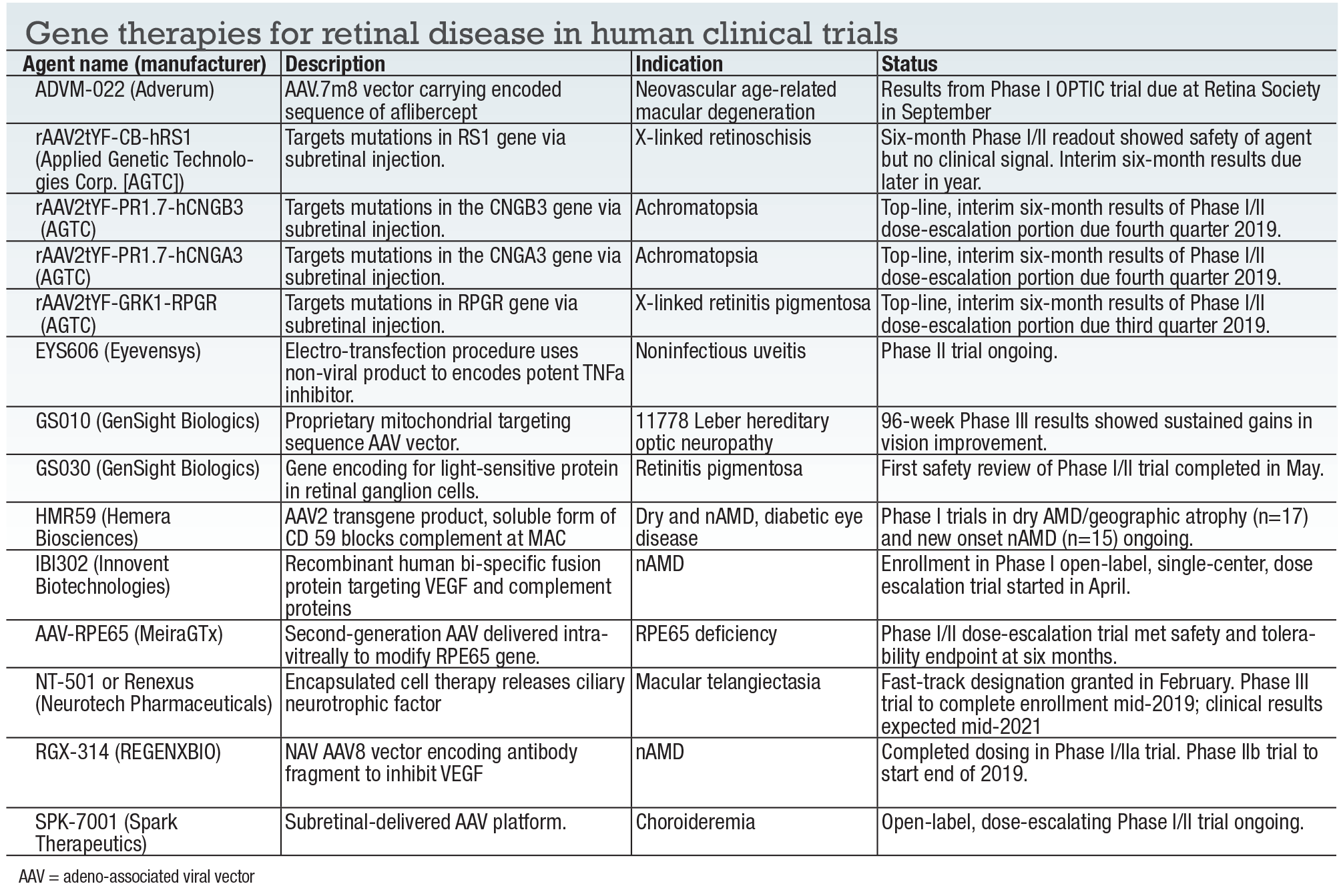
This report focuses on gene therapy programs in human clinical trials (Table, page 42). A few companies not mentioned here, including 4D Molecular Therapies and
IVERIC bio, formerly known as Ophthotech, have ophthalmic programs in the preclinical stage. And in 2016 Allergan acquired Retro-
Sense Therapeutics, which had been developing RST-001, an optogenetic platform for retinitis pigmentosa, but no progress has been reported since. The list we provide here is not all-inclusive, but reports on programs that have publicized readouts in the past year or have had active trials through that time.
Phase III trials
• GenSight Biologics. The REVERSE Phase III clinical trial of GS010 enrolled 37 subjects who had visual loss due to the 11778-ND4 form of Leber hereditary optic neuropathy (LHON) that occurred in the previous six to 12 months. At 96 weeks, GS010-treated eyes showed a mean improvement equivalent to 15.4 letters or 3 lines, sustaining improvements reported in 48- and 72-week readouts. GS010 isn’t GenSight’s only rodeo. It’s also evaluating GS030 for retinitis pigmentosa (Phase I/II), and is doing preclinical work on a vector for the ND form of LHON and a treatment for dry AMD and geographic atrophy.
• Neurotech Pharmaceuticals. NT-501, also known as Renexus, is an encapsulated cell therapy for treatment of macular telangiectasia. Phase II results, published in the journal Ophthalmology,1 reported NT-501 treatment slowed the progression of retinal degeneration compared to the sham group. The study also documented that treated patients stabilized their reading speeds. NT-501 is a novel cell-based drug-delivery system in which human-derived cells encapsulated in a semipermeable hollow fiber membrane device release ciliary neurotrophic factor (CNTF). Patients from Phase I/II studies are being followed to evaluate the long-term effects of CNTF on retinal degeneration.
Programs in AMD
• Adverum Biotechnologies. The program to develop AADVM-022 for treatment of nAMD took a step forward in May when the Food and Drug Administration lifted the clinical hold on dosing of the second cohort of the OPTIC Phase I trial. This cohort is to receive a dose three-times greater than that used in the first cohort (n=6), although the initial dose in the second cohort will actually be three times lower. ADVM-022 is a proprietary vector capsid, AAV.7m8, that carries an aflibercept coding sequence to provide sustained dosing. Adverum received fast-track designation for ADVM-022 last year. The company expects to present interim 24-week data from the OPTIC trial at the Retina Society meeting in September.
• REGENXBIO. RGX-314 is being developed as a potential one-time subretinal treatment for wet AMD and diabetic retinopathy. It uses the proprietary NAV technology with the AAV8 vector encoding an antibody fragment to inhibit vascular endothelial growth factor. The Phase I/IIa trial has completed dosing in all five cohorts. An interim readout reported that patients in one cohort had a durable treatment at one year after a single administration of RGX-314 after pretreatment. Top-line data from the trial, including the fourth and fifth cohorts, is due by year end.
• Hemera Biosciences. HMR59 is a soluble form of CD59, the protective protein normally found on the cellular plasma membrane. Intended to be a one-time intravitreal treatment for both wet and dry AMD, it’s in Phase I trials for both indications. Eighteen-month data from the dry AMD study is expected at an upcoming meeting.
• Innovent Biologics. This Suzhou,
China-based company initiated enrollment this spring in a Phase I trial of IBI302, a novel recombinant fully human bi-specific fusion protein targeting both VEGF and complement proteins in nAMD. Like Hemera’s HMR59, the goal is to develop a one-time intravitreal treatment for the disease.
 |
Other noteworthy programs
At least four other companies are pursuing gene therapies for other indications. Two worth mentioning are:
• Spark Therapeutics. An open-label, dose-escalating Phase I/II trial of investigational SPK-7001 for treatment of x-linked choroideremia (CHM) is ongoing.
• Applied Genetics Technologies Corp. AGTC may have the most robust pipeline, although it encountered a setback late last year when Biogen terminated a collaboration agreement. AGTC has four adeno-associated virus (AAV)-based gene therapies in human trials, including rAAV2tYF-CB-hRS1, an intravitreal injection for X-linked retinoschisis due to mutations in the RS1 gene. Results late last year showed that the agent is generally safe and well-tolerated, but showed no signs of clinical activity at six-months. AGTC is monitoring enrolled patients, with interim six-month data of dose-escalation and dose-expansion groups due in the third and fourth quarters, respectively.
REFERENCE
1. Chew EY, Clemons TE, Jaffe GJ, et al, for the Macular Telangiectasia Type 2-Phase 2 CNTF Research Group. Effect of ciliary neurotrophic factor on retinal neurodegeneration in patients with macular telangiectasia type 2: A randomized clinical trial. Ophthalmology. 2019;126:540-549.




