Take-home Points
|
 |
| Grayson W. Armstrong, MD, MPH |
|
Bios Dr. Armstrong is a physician and surgeon at Massachusetts Eye and Ear and an instructor in ophthalmology at Harvard Medical School, Boston. DISCLOSURE: Dr. Armstrong is co-founder of Ocular Technologies, a tele-ophthalmology device company. |
Artificial intelligence has received considerable attention for its promise and potential in advancing the field of retina, and for good reason. There have been remarkable breakthroughs in the last few years in the use of AI to screen for, diagnose, triage and grade ophthalmic disease. This has culminated in Food and Drug Administration approval of two autonomous AI devices meant to screen for diabetic retinopathy.
Additional AI-based retinal diagnostics are under investigation and stand to enter the U.S. market in the near future. What’s more, AI has been applied to the detection of systemic disease through the use of ophthalmic imaging.
While research applications of AI continue to advance at breakneck speeds, clinical uptake is proceeding at a more metered pace. Ultimately, retina specialists, when looking to implement AI in their practices or referral networks, must consider issues related to patient and physician perceptions of AI, privacy concerns and practical considerations such as billing and regulation.
What AI is
AI is a type of engineering that allows computers to perform intelligent functions. Machine learning (ML) is a subset of AI that enables computers to evaluate and learn from a set of data and subsequently perform a task to autonomously classify future data and draw conclusions about the data. The performance of an ML algorithm can be evaluated using metrics such as sensitivity, specificity, accuracy and area under the receiver operating characteristic (AUROC).
Clinical images are particularly useful to input as data into ML models and often require specific types of ML algorithms, known as convolutional neural networks (CNNs), to evaluate the images and make conclusions. CNNs operate like the complex neuronal synapses in the brain or retina. They relay signals from one input down a chain of multiple interconnected neurons that ultimately outputs a conclusion about the initial input (Figure 1). Conclusions can include results of disease screening, diagnosis or disease grading and classification.1
Retina has an abundance of imaging modalities at our disposal. While fundus photography and fluorescein angiography have been incorporated into clinical practice for years, newer modalities such as optical coherence tomography and OCT angiography have experienced rapid uptake. The prevalence of clinical images, and the ability of these images to directly assess neuronal and vascular tissue, makes retina an ideal field to take advantage of the promise of AI.
AI in disease detection
The prevalence of age-related and comorbid ophthalmic conditions is rising as the population ages. Worldwide, the number of people with diabetes is estimated to increase from 415 million today to 642 million by 2040.2 Each patient with diabetes will require a diabetic eye screening to detect treatable disease, although fewer than half of them are currently screened annually.3,4
Age-related macular degeneration is already the leading cause of central vision loss in the United States, and the worldwide prevalence is estimated to rise from 196 million in 2020 to 288 million by 2040.4 This increase in retinal pathology will drive utilization of ophthalmic care services in the United States and elsewhere, but the number of retina specialists isn’t expected to increase commensurately.5,6 This increased demand for eye care, along with technological advancements in the field of ophthalmology and computer science, has led to a greater focus on AI.
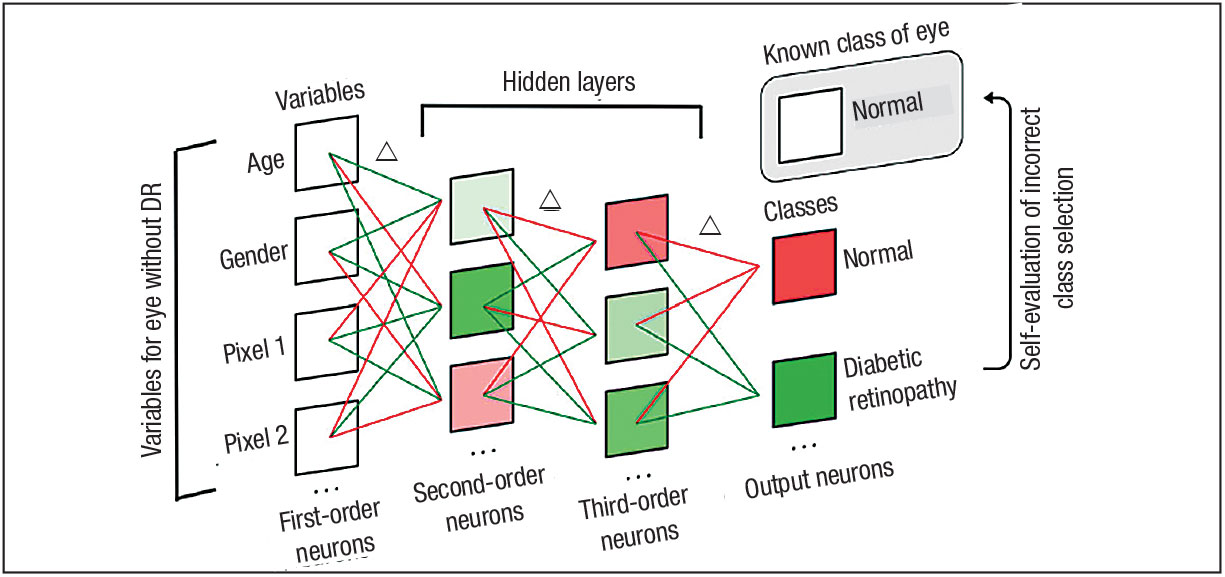 |
| Figure 1. A model neural network model showing incorrect class selection and subsequent weighted connection modifications.1 A neural network is composed of multiple neuronal layers. The first-order neurons represent clinical variables for an eye without diabetic retinopathy. Weighted connections are represented as positively weighed (green) or negatively weighted (red) lines. The second- order and third-order neurons comprise the hidden layers. The algorithm has output a class selection of “diabetic retinopathy,” which is incorrect. The algorithm compares the output of “diabetic retinopathy” to the known class of the eye, “normal.” Since the output was incorrect, the algorithm determines which weighted neuronal connections to modify (indicated by Δ). (Used with permission of Wolters Kluwer Health) |
ML algorithms have shown incredible promise in the detection of diseases such as DR and AMD.7,8 Beyond this, AI algorithms have also shown proficiency in grading disease and properly discriminating patients with mild disease from those with referable disease.8-10 These technological advances stand to offload screening exams from ophthalmologists to computer-based screening programs, which will require lower-compensated and less-skilled operators. As routine screening exams are increasingly relegated to AI-enabled machines and cameras, patient referrals to practicing retina specialists will inevitably increase with a commensurate escalation in the demand for treatment of vision-threatening disease.
Predicting treatment needs
AI-based algorithms have also demonstrated the ability to predict the need for treatment in various patient populations. For example, algorithms can utilize OCT images to determine which patients with AMD will require anti-VEGF injections.11 Patients with diabetes also benefit from similar algorithms that can predict who will need laser treatment, anti-VEGF injections or surgery. 12
Predicting patient outcomes in retinal disease has also been assessed using AI
algorithms. Using OCT images from patients on anti-VEGF therapy for neovascular AMD, multiple studies have found that a patient’s future visual acuity can be predicted with relative certainty.13,14 The ability to prognosticate could help in patient counseling and encouraging medication adherence.
Lastly, retinal imaging has been used to assess nonophthalmic disease indicators and overall patient health. One of the earliest studies to use fundus photography to assess patient health demonstrated an ability to predict a patient’s gender, smoking status, systolic blood pressure and other metrics.15 More recent studies have shown an ability to detect Alzheimer’s disease,16,17 renal function18 and autism spectrum disorder19 (Figure 2). While the research in these areas is still early, there’s vast potential to improve patient care and detect disease early with non-invasive retinal imaging.
Clinical readiness
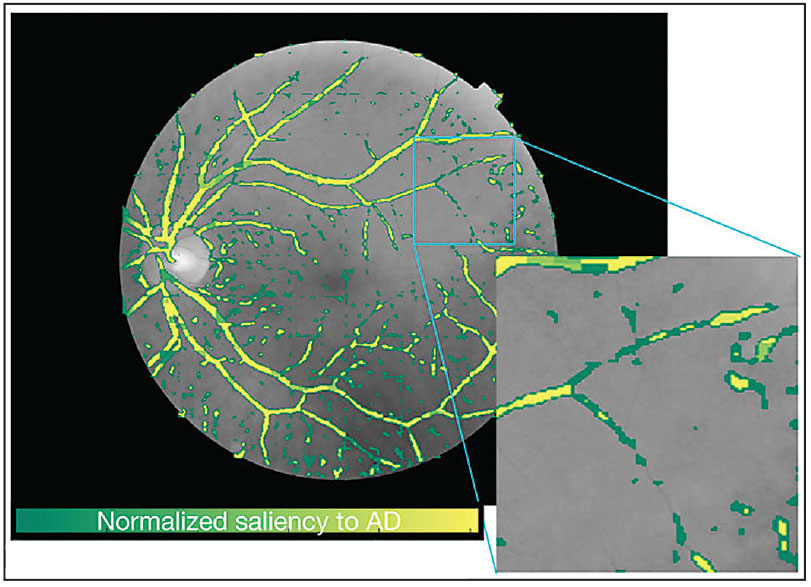 |
| Figure 2. Generated saliency map of a fundus photograph after the use of artificial intelligence for the detection of Alzheimer’s disease in a patient.17 A general observation that researchers can make through the saliency map is that small vessels contribute more than major vessels to Alzheimer's disease classification. Green pixels are more salient for classifying Alzheimer’s disease while yellow pixels don’t significantly contribute to this classification. According to this saliency map, small vessels and capillary vessels are more important in determining whether this image belongs to an Alzheimer’s disease subject. (Image used under creative commons license. Source: Science Reports) |
While the scientific community seems to be publishing results of new and innovative retinal image-based AI algorithms for disease detection, triage and prognostication at a breakneck pace, commercial applications of these technologies is lagging behind. A few standout retinal imaging technologies incorporating AI have undergone FDA approval and are now on the market.
In the United States, IDx-DR (Digital Diagnostics) was the first autonomous AI technology approved for use in detection of pathology without the need for a physician to review the images.20 Marketed for use in primary-care offices and other non-ophthalmic outpatient settings with patients with diabetes, the technology can autonomously, through the use of a Topcon NW400 fundus camera, differentiate patients with referable DR, defined as moderate DR or macular edema, from those with non-referable DR. Based on the autonomous read of the fundus photograph, the program can recommend that the physician capturing the image refer the patient to an ophthalmologist when appropriate.
Retina specialists will probably not purchase this AI tool for use in their own offices, but its implementation in integrated health systems or local referral networks stands to increase both the number of patients with DR referred to retina specialists and the percentage of patient referrals with pathologic findings requiring treatment.
This autonomous system allows patients with diabetes to obtain ophthalmic care without the need to visit an ophthalmologist and thus improve access to care, while increasing the proportion of a primary-care physician’s patient panel that achieves the quality metric of obtaining an annual diabetic screening exam. The cost of the IDx-DR system, which includes the software and the Topcon camera, may be prohibitive for small primary-care practices, but bigger practices caring for a large proportion of patients with diabetes could achieve cost-effectiveness.
Recently, a second autonomous AI screening technology for DR was approved, EyeArt (Eyenuk). While the EyeArt program can detect more-than-mild DR similar to IDx-DR, it also boasts the ability to detect vision-threatening DR. The system performed remarkably well, with the company reporting a sensitivity of 96 percent and specificity of 88 percent in detecting referable DR, and sensitivity of 92 percent and specificity of 94 percent for detecting vision threatening DR.21 Real-world trials of the technology have found similar accuracy in detecting DR.22 Similar to IDx-DR, this technology is beneficial for patients with diabetes in primary-care practices.
AI technology on the horizon
Additional AI-based technologies are being evaluated that could significantly influence the future of retina practice. Notal Vision is developing a home-based OCT diagnostic device intended to be used by patients with nAMD. While this device is not yet FDA approved, it’s in clinical trials and stands to improve remote patient monitoring and earlier detection of pathologic changes for patients with AMD, thus enabling retina specialists to treat disease earlier in its course and intervene before significant fibrosis and permanent vision loss occurs.
Additional products are undoubtedly in the pipeline, including a result of a recently announced collaboration between Novartis Pharma AG and RetinAI in assessing disease severity in patients with neovascular AMD.23
Privacy and regulation considerations with AIWhile artificial intelligence-based systems currently on the market don’t transmit protected health information or clinical images off-site to enable the algorithm to detect evidence of diabetic retinopathy, physicians should be mindful that future AI technologies may not operate similarly. Clinical images of the retina should be stored securely and, when desired, should be transmitted between providers securely. Protection of patient privacy is paramount, and data security violations can result in significant fines or litigation. State regulations require that physicians providing patient care be licensed to practice medicine within that jurisdiction. Lastly, antikickback statues prohibit physicians from referring patients to entities with which they have a financial relationship. This can complicate referral relationships if a retina practice provides an AI-based technology to the referring clinician and is subsequently sent patients. The complexity of these issues make it prudent to seek professional counsel before you implement AI-based technologies and screening programs. |
Clinical workflow
Today the only retina-based AI technologies approved by the FDA are devoted to DR screening. Incorporating these novel technologies into existing workflows requires a coordinated effort between retina specialists and non-retina providers caring for patients with diabetic disease.24 If the technology is to be successful, it should be implemented in patient populations and care sites with a high prevalence of diabetes. Personnel will need to be tasked with identification of patients with diabetes and coordination of fundus photography images.
Because high-quality fundus photographs are required for the AI to operate effectively, staff will need training in proper operation of the fundus camera and should maintain their skills by using the camera frequently.
Once the images are captured and the report generated, patient education on the findings is necessary to encourage proper follow-up with a retina specialist. Coordinated referral placement should ensure ease of access for patients.
Financial sustainability of any DR screening program, including those using AI-based technologies, requires careful consideration of billing and reimbursement. Fortunately, a novel CPT code, 9225X, has been created to accommodate AI-based retinal screening platform utilization.25
As increasingly varied AI-based retinal technologies enter the market, physicians will need to make similar considerations and carefully incorporate the tools into existing workflows. There will be no one-size-fits-all approach. Due to cost considerations, varying prevalence of diseases in target populations and heterogenous clinical practice patterns, different health-care systems and physicians will need to implement the technologies differently.
Bottom line
AI has entered the mainstream and is ready to be incorporated into retina referral networks. Current FDA-approved, AI-based devices are meant to screen for referable DR and vision threatening DR in primary-care clinics, but products on the horizon will go well beyond DR screening and could be integrated into retina practices, be used by referring providers, or provide remote monitoring in patients’ homes.
Ongoing research shows promising results in retinal and systemic disease detection, grading and prognostication. In time, these AI algorithms will enter clinical practice and fulfill the potential of AI-based retinal imaging in advancing patient care. RS
REFERENCES
1. Armstrong GW, Lorch AC. A(eye): A Review of current applications of artificial intelligence and machine learning in ophthalmology. Int Ophthalmol Clin. 2020;60:57-71.
2. Ogurtsova K, da Rocha Fernandes JD, Huang Y, et al. IDF Diabetes Atlas: Global estimates for the prevalence of diabetes for 2015 and 2040. Diabetes Res Clin Pract. 2017;128:40-50.
3. Murchison AP, Hark L, Pizzi LT, et al. Non-adherence to eye care in people with diabetes. BMJ Open Diabetes Res Care. 2017;5:e000333.
4. Wong WL, Su X, Li X, et al. Global prevalence of age-related macular degeneration and disease burden projection for 2020 and 2040: A systematic review and meta-analysis. Lancet Glob Health. 2014;2:e106-e116.
5. Feng PW, Ahluwalia A, Feng H, Adelman RA. National trends in the United States eye care workforce from 1995 to 2017. Am J Ophthalmol. 2020;218:128-135.
6. Gibson DM. The local availability of eye care providers and the vision health of adults in the United States. Ophthalmic Epidemiol. 2016;23:223-231.
7. Kermany DS, Goldbaum M, Cai W, et al. Identifying medical diagnoses and treatable diseases by image-based deep learning. Cell. 2018;172:1122-1131.e9.
8. Ting DSW, Cheung CY, Lim G, et al. Development and validation of a deep learning system for diabetic retinopathy and related eye diseases using retinal images from multiethnic populations with diabetes. JAMA. 2017;318:2211-2223.
9. Burlina P, Pacheco KD, Joshi N, Freund DE, Bressler NM. Comparing humans and deep learning performance for grading AMD: A study in using universal deep features and transfer learning for automated AMD analysis. Comput Biol Med. 2017;82:80-86.
10. Gulshan V, Peng L, Coram M, et al. Development and validation of a deep learning algorithm for detection of diabetic retinopathy in retinal fundus photographs. JAMA. 2016;316:2402-2410.
11. Prahs P, Märker D, Mayer C, Helbig H. Deep Learning zur Unterstützung der Therapieentscheidung bei intravitrealen Injektionen [Deep learning to support therapy decisions for intravitreal injections]. Ophthalmologe. 2018;115:722-727.
12. Takahashi H, Tampo H, Arai Y, Inoue Y, Kawashima H. Applying artificial intelligence to disease staging: Deep learning for improved staging of diabetic retinopathy. PLoS One. 2017;12:e0179790.
13. Aslam TM, Zaki HR, Mahmood S, et al. Use of a neural net to model the impact of optical coherence tomography abnormalities on vision in age-related macular degeneration. Am J Ophthalmol. 2018;185:94-100.
14. Schmidt-Erfurth U, Bogunovic H, Sadeghipour A, et al. machine learning to analyze the prognostic value of current imaging biomarkers in neovascular age-related macular degeneration. Ophthalmol Retina. 2018;2:24-30.
15. Poplin R, Varadarajan AV, Blumer K, et al. Prediction of cardiovascular risk factors from retinal fundus photographs via deep learning. Nat Biomed Eng. 2018;2:158-164.
16. Lemmens S, Van Craenendonck T, Van Eijgen J, et al. Combination of snapshot hyperspectral retinal imaging and optical coherence tomography to identify Alzheimer's disease patients. Alzheimers Res Ther. 2020;12:144.
17. Tian J, Smith G, Guo H, et al. Modular machine learning for Alzheimer's disease classification from retinal vasculature. Sci Rep. 2021;11:238.
18. Sabanayagam C, Xu D, Ting DSW, et al. A deep learning algorithm to detect chronic kidney disease from retinal photographs in community-based populations. Lancet Digit Health. 2020;2:e295-e302.
19. Lai M, Lee J, Chiu S, et al. A machine learning approach for retinal images analysis as an objective screening method for children with autism spectrum disorder. E Clin Med. 2020;28:100588.
20. Abràmoff MD, Lavin PT, Birch M, Shah N, Folk JC. Pivotal trial of an autonomous AI-based diagnostic system for detection of diabetic retinopathy in primary care offices. NPJ Digit Med. 2018;1:39.
21. Lim J, Bhaskaranand M, Ramachandra C, Bhat S, Solanki K, Sadda S. Artificial intelligence screening for diabetic retinopathy: analysis from a pivotal multi-center prospective clinical trial. Paper presented at: ARVO Imaging in the Eye Conference; April 27, 2019; Vancouver, BC, Canada.
22. Bhaskaranand M, Ramachandra C, Bhat S, et al. The value of automated diabetic retinopathy screening with the EyeArt system: A study of more than 100,000 consecutive encounters from people with diabetes. Diabetes Technol Ther. 2019;21:635-643.
23. RetinAI announces collaboration with Novartis to provide artificial intelligence solutions in ophthalmology [press release]. RetinAI, Bern, Switzerland. December 8, 2020. https://www.retinai.com/press-releases Accessed February 12, 2021.
24. Horton MB, Brady CJ, Cavallerano J, et al. Practice guidelines for ocular telehealth-diabetic retinopathy, third edition. Telemed J E Health. 2020;26:495-543.
25. American Medical Association. CPT Editorial Summary of Panel Actions May 2019. August 26, 2019. https://www.ama-assn.org/system/files/2019-08/may-2019-summary-panel-actions.pdf Accessed February 12, 2021.



