 |
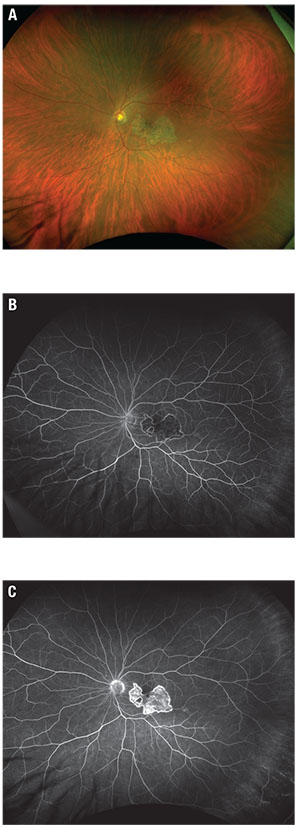
| Figure 1. Fundus photograph (A) shows a placoid lesion of retinal pigment epithelium atrophy in the central macula. Early phase fluorescein angiography (B) shows hypofluorescence at the site of the lesion and late-phase FA (C) shows staining. |
Examination
Best-corrected visual acuity was 20/15-1 and 20/70-1, with intraocular pressures of 13 mmHg and 15 mmHg in the right and left eyes, respectively. A trace afferent pupillary defect was present in the left eye. Extraocular muscle motility and confrontational visual fields were full in both eyes. No anterior chamber or anterior vitreous cells were visible on anterior segment exam.
Dilated fundus exam was unremarkable in the right eye but the left eye showed a 5-disc-area placoid lesion of RPE atrophy in the central macula with a small round hypopigmented lesion along the inferotemporal arcade (Figure 1A). No Vascular sheathing, hemorrhages or subretinal fluid were present.
Workup and Diagnosis
Fluorescein angiography of the left eye (Figures 1B and C) was notable for early hypofluorescence of the lesion with hyperfluorescent borders. We also noted late staining of the lesion with central mottling.
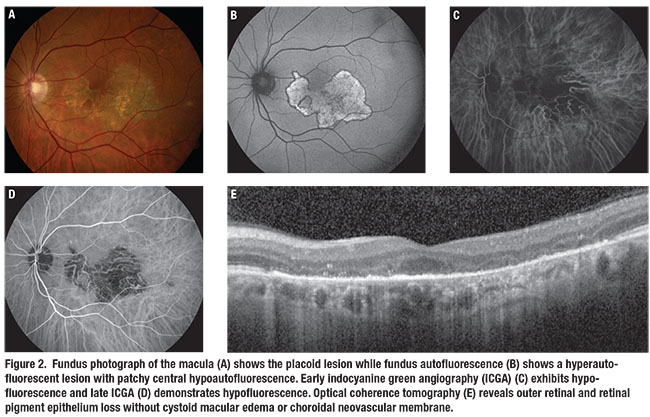
Fundus autofluorescence showed a hyperautofluorescent lesion with hypoautofluorescent borders (Figure 2B). Indocyanine green angiography showed early and late hypoperfusion with loss of choriocapillaris in the central macula corresponding to the lesion (Figures 2C and D). Spectral-domain optical coherence tomography revealed well-demarcated outer retinal and RPE loss corresponding to the lesion without cystoid macular edema or choroidal neovascularization (Figure 2E).
The laboratory workup included a complete blood count, which was normal, and a complete metabolic panel. HIV antibodies, syphilis titers and quantiferon gold testing were negative. Given the serpiginous appearance of the central placoid lesion with a negative infectious workup and characteristic features, including early hypofluorescence and late hyperfluorescence on FA and hyperautoflorescence on FAF, we diagnosed macular serpiginous choroiditis.
Treatment Plan
We initiated a trial of systemic corticosteroids with prednisone 60 mg daily. At a follow-up examination two weeks later, the patient noted subjective improvement in central scotoma size. On clinical exam we observed a stable lesion appearance, also evident on repeat FAF and FA imaging. Given the concerning finding of hyperautofluorescence of the lesion with risk of disease progression and fellow-eye involvement, we decided to initiate immunomodulatory therapy with mycophenolate mofetil and slowly taper the systemic corticosteroids.
Discussion
Serpiginous choroiditis (SC) is a chronic, asymmetrically bilateral inflammatory posterior uveitis and one of the white dot syndromes. It is characterized by recurrent inflammation of the outer retina, RPE and choriocapillaris.1 Serpiginous choroiditis typically presents in the fourth to sixth decade of life and affects men more than women.1-3 It is thought to be a rare condition. While actual prevalence in the United States is unknown, an epidemiologic study in India found SC in up to 18 percent of uveitis referrals.4
Patients often complain of decreased vision, central scotoma and metamorphopsia. Lesions classically appear in a peripapillary distribution and extend in a serpentine pattern throughout the posterior pole. Minimal associated vitritis, vasculitis or anterior segment inflammation is present. Initially active lesions are yellowish-gray and, when left untreated, can progress to choroidal and RPE atrophy with mottled pigmentary changes.1-3
While the underlying etiology of SC remains elusive, autoimmune, vascular and infectious causes are implicated. Mycobacterium tuberculosis is a well-recognized entity that produces serpiginous-like choroiditis. In fact, studies have shown an association between positive tuberculin skin test and SC, which may indicate a common underling infectious etiology.
Literature from TB-endemic areas has reported clinical characteristics of this TB-related “serpiginous-like choroiditis,” or “multifocal serpiginoid choroiditis” to better distinguish it from more classic SC. These characteristics include a greater preponderance of vitreous inflammation, lesions of a more multifocal pattern or that favor the macula rather than the peripapillary region early on, and a positive response to a full course of antitubercular treatment (ATT).
In these retrospective studies, treatment response to ATT would occur in some cases with or without concomitant corticosteroid therapy, and often without the use of immunosuppression.5,6 As such, the exclusion of TB is recommended in every case due to the significantly different treatment approaches.
The diagnosis is clinical. However, an investigative workup is mandatory to rule out infectious SC-mimicking conditions such as TB, sarcoidosis, syphilis and herpetic infection.
 |
Other Forms of SC
Besides the classic peripapillary form of SC, two other important clinical variants exist. Ampiginous choroiditis, also referred to as persistent placoid chorioretinitis, represents a hybrid between acute posterior multifocal placoid pigment epitheliopathy (APMPPE) and SC.7 Lesions are multifocal and occur in both the posterior pole and periphery, but unlike APMPPE the pigment changes and RPE atrophy remain after lesions resolve.1,8
With the macular serpiginous variant, as reflected by the name, the lesions preferentially occur in the macula and may not involve the juxtapapillary retina. Characteristics of this variant are a worse prognosis due to foveal involvement, a destructive course and high frequency of choroidal neovascular membrane formation.2,3 Because the lesion we describe here exclusively involves the central macula, this is a case of the macular serpiginous variant.
 |
Treatment is usually initiated with systemic corticosteroids with or without topical or periocular steroids. Because SC is almost always a bilateral and progressive disease, addition of immunomodulatory agents results in better long-term control. Antimetabolites and/or T-cell inhibitors are effective for suppressing the inflammation SC causes. For recalcitrant disease, alkylating agents and biologics can be employed.3,9 Long-term follow-up is important to monitor response to treatment and detect recurrences. RS
REFERENCES
1. McCannel CA, ed. 2015-2016 Basic and Clinical Science Course (BCSC), Section 12: Retina and Vitreous. San Francisco, CA: American Academy of Ophthalmology; 2015.
2. Annamalai R, Sudharshan S, Biswas J. Clinical features, investigations, management, and prognosis of serpiginous choroiditis. Asia Pac J Ophthalmol. 2012;1:287-295.
3. Khanamiri HN, Rao NA. Serpiginous choroiditis and infectious multifocal serpiginoid choroiditis. Surv Ophthalmol. 2013;58:203-232.
4. Blumenkranz MS, Gass JD, Clarkson JG. Atypical serpiginous choroiditis. Arch Ophthalmol. 1982;100:1773–1775.
5. Gupta V, Gupta A, Arora S, Bambery P, Dogra MR, Agarwal A. Presumed tubercular serpiginous-like choroiditis: clinical presentations and management. Ophthalmology. 2003;110:1744-1749.
6. Bansal R, Gupta A, Gupta V, Dogra MR, Sharma A, Bambery P. Tubercular serpiginous-like choroiditis presenting as multifocal serpiginoid choroiditis. Ophthalmology. 2012;119:2334-2342.
7. Lambrecht P, Claeys M, Schryver ID. A case of ampiginous choroiditis. Case Rep Ophthalmol. 2015;6:453-457.
8. Golchet PR, Jampol LM, Wilson D, Yannuzzi LA, Ober M, Stroh E. Persistent placoid maculopathy. Ophthalmology. 2007;114:1530-1540.
9. Hooper PL, Kaplan HJ. Triple agent immunosuppression in serpiginous choroiditis. Ophthalmology. 1991;98:944-951.



