Even with the launch of the first two anti-VEGF biosimilars last year, retina specialists seem somewhat ambivalent about using these copy-cat versions of tried and true treatments, a new report based on a survey of ophthalmologists has found.
The report, titled “Special Topix: Ophthalmology Biosimilars Today and Tomorrow,” is based on a survey of 80 U.S. ophthalmologists conducted in November 2022. Spherix Global Insights, a consultancy to the life sciences industry, released the report in December 2022.
 |
|
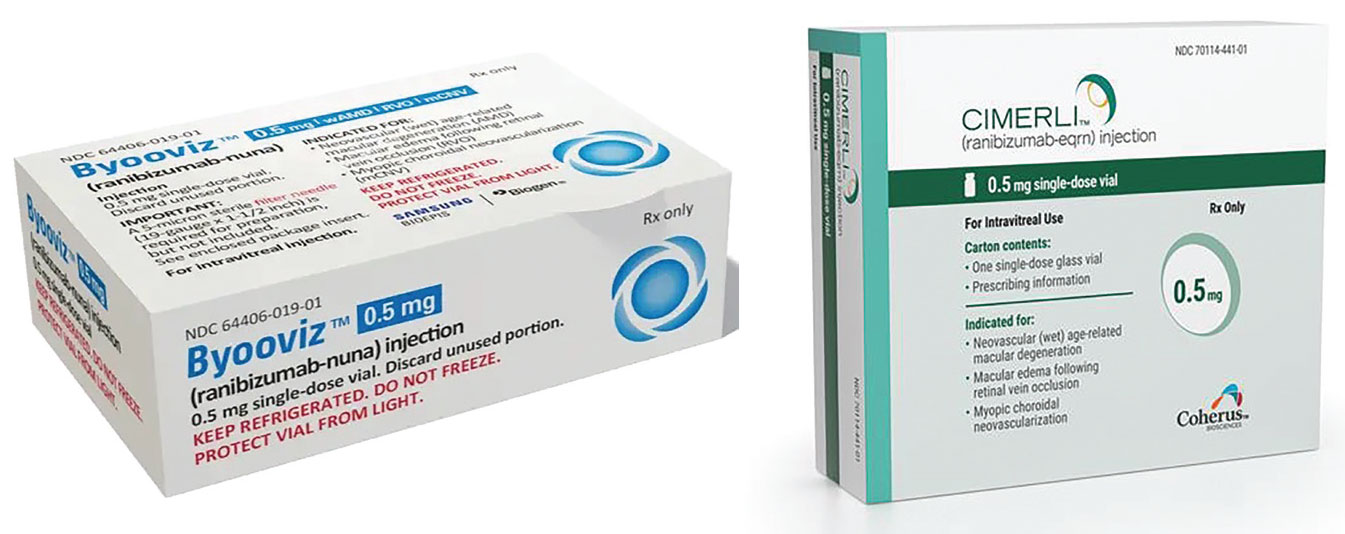
These two haven't done much to raise awareness about biosimilars among retina specialists, a recently released market study found. |
Chrystal Ferguson, franchise head, ophthalmology, summarizes the key findings: “U.S. ophthalmologists are definitely open to biosimilars, in theory, at this stage, but they have reservations and concerns in practice.”
The report gauged movement in ophthalmologists’ attitudes toward biosimilars since a December 2021 survey, finding that while familiarity with biosimilars increased in that period, their comfort level with the clinical evidence required for approval actually declined.
In Brief More than a quarter of U.S. adults age 71 and older had vision impairment in 2021, according to an analysis of the 2021 National Health and Aging Trends Study published online in JAMA Ophthalmology (2023;doi:10.1001/jamaophthalmol.2022.5840). A higher prevalence of vision impairment was associated with older age, lower education and income, nonwhite race and Hispanic ethnicity. The Food and Drug Administration approved the ML6710i photodynamic laser for equivalent use with Visudyne (verteporfin for injection, Bausch + Lomb) photodynamic therapy for treatment of predominantly classic subfoveal choroidal neovascularization due to age-related macular degeneration. The ZETA-1 Phase II trial of APX3330 in diabetic retinopathy didn’t meet the primary endpoint of a having an unspecified percentage of patients achieve a more than two-step improvement in Diabetic Retinopathy Severity Scale at week 24, according to topline results Ocuphire Pharma released. However, the company says additional efficacy endpoints were “directionally favorable.” Further results are due in February. |
Unease with ‘extrapolation’
"Being on the biosimilar pathway for approval through the Food and Drug Administration allows a product to go through what’s called extrapolation, which means the actual clinical trial piece can be conducted in one particular indication and then those findings are extrapolated to the other indications that the reference product is approved for,” Ms. Ferguson says. “So as the ophthalmologists are learning more about this process, they are still feeling a little uneasy with that concept.”
The report found that the launches of Byooviz (Biogen) and Cimerli (Coherus Biosciences), both Lucentis (ranibizumab) biosimilars, didn’t have much impact on raising awareness about the treatments, according to almost half of the ophthalmologists surveyed. The vast majority of survey respondents—84 percent—were retina specialists, with the rest being general ophthalmologists.
The launch of the two biosimilars has made more ophthalmologists aware of the category, Ms. Ferguson says. “Having Byooviz and Cimerli now on the market has really enabled them to have that conversation and start to dig and to better understand what a biosimilar is and how it can help their patients,” she says.
She acknowledges that retina specialists are prone to “wait and see,” alluding to the experience with the launch of Beovu (brolucizumab), which Novartis pulled back on in 2020 after reports came out linking the drug to cases of ocular inflammation and vasculitis.
“The Beovu experience of a couple years ago has really sort of hindered a rapid-adoption mindset,” Ms. Ferguson says. “So, I think, at this point, the fact that they are available is definitely helping to broaden the education of the community, but isn’t necessarily at a point yet where it’s tipping attitudes.”
The allure of Eylea
An Eylea biosimilar is likely to have broader appeal, the survey found. “I think that what it comes down to is that Eylea gets wider use at this point,” she says. “We’ve spoken to several physicians that have said, ‘If there had been a Lucentis biosimilar back in the Lucentis heyday, I would’ve been all over it, but my Lucentis use has actually decreased over the years.’”
But even at that, ophthalmologists’ knowledge about the development of Eylea biosimilars “is pretty minimal,” Ms. Ferguson says. “We have surveyed the physicians on the specific aflibercept biosimilars that are in development, and there is very low awareness, very low familiarity with any specific one in development.”
Another barrier to retina specialists embracing biosimilars is that they don’t know the companies sponsoring trials. “So, while that’s not necessarily a primary barrier, Genentech and Regeneron, Lucentis and Eylea manufacturers, have been household names for years,” she says. “So there is a little bit of a latent effect in terms of being able to rely on these unknown manufacturers as partners to the practice and to the patient.”
Further along, payers will probably have a key role in driving demand for biosimilars in retina. “There’s going to be a really fine line between incentivizing prescribers to use biosimilars and then also putting them off by mandating they prescribe biosimilars,” Ms. Ferguson says.
What could ‘shake’ market
As retina biosimilars evolve, two events could “shake” the market, in her words: the 8-mg high-dose
aflibercept, now in clinical trials (the Eylea biosimilars reference the 2-mg formulation); and Outlook Therapeutics’ program to develop an ophthalmic formulation of Avastin, typically the first-step therapy in drug formularies. The latter has an FDA action date for possible approval in the summer. Eylea comes off patent later this year.
As more biosimilars get approved, ophthalmologists’ knowledge and comfort level with them may pick up. However, the report specifically noted that most ophthalmologists are unaware that Viatris, a Mylan company, filed for an FDA review of M710/MYL-1701P, its aflibercept biosimilar. The survey found that ophthalmologists aren’t likely to embrace an aflibercept biosimilar and even less likely if high-dose aflibercept gets approved.
“Having more affordable treatments is definitely a welcome thing in the ophthalmologist community, but there really just needs to be a lot more personal experience in order to be able to increase that comfort level, and there are several other things in the pipeline that could potentially change that calculus,” Ms. Ferguson says.
The Spherix report is available at www.spherixglobalinsights.com.
— Richard Mark Kirkner



