 |
Studies have demonstrated the presence of VEGF in fibrovascular membranes in eyes with diabetic retinopathy,4 confirmed hypoxia stimulated secretion of VEGF in retinal pigment epithelium (RPE) cells,5 and identified subsequent reduction in VEGF mRNA at a cellular level with normal oxygenation.3 Retinal hypoxia contributes to the pathogenesis of diabetic macular edema (DME) and diabetic retinopathy (DR), and VEGF has been implicated as an important therapeutic target in DME and DR.6,7 In addition, VEGF has been targeted in other retinal vascular diseases, such as retinal vein occlusive disease.8–11
The Anti-VEGF Effect
The introduction of novel VEGF antagonists has revolutionized the management of diabetic retinopathy with macular edema and retinal vein occlusion with macular edema.12,13 Phase III trials of anti-VEGF therapy have demonstrated improved diabetic retinopathy scores, decreased macular edema, gain of visual acuity and reduced progression to neovascularization.8,14,15 Aflibercept (Eylea, Regeneron Pharmaceuticals) showed greater than two-step improvement in Diabetic Retinopathy Severity Scale (DRSS) scores compared to laser, highlighting the role that anti-VEGF plays in regression of diabetic retinopathy.14,16
RISE and RIDE trials similarly showed
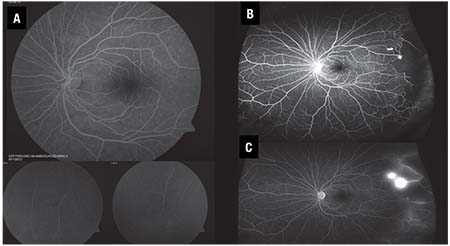 |
| Figure 1. Regular fluorescein angiography (FA) (A) did not show the peripheral nonperfusion and neovascularization highlighted on widefield FA (B, C). |
In the VIBRANT study for branch retinal vein occlusion (BRVO), a direct comparison of laser treatment vs. anti-VEGF, demonstrated not only a greater reduction of macular edema in the anti-VEGF group but improved perfusion of the retina with less than 10 disc areas of capillary nonperfusion.8 CRUISE (central retinal vein occlusion) and BRAVO (branch retinal vein occlusion) clinical trials demonstrated improved visual acuity and macular edema in the ranibizumab arms.9,10 SCORE2 similarly showed that eyes treated with bevacizumab or aflibercept had improvement in visual acuity and macular edema in CRVO and hemiretinal vein occlusion.11
The COPERNICUS study for central retinal vein occlusion (CRVO) showed no progression to neovascularization in the VEGF trap-eye group compared to 7 percent in sham-treated eyes.15 The authors postulated that anti-VEGF may allow reperfusion by inhibiting leukostasis and retinal ischemia triggered by VEGF. While many studies have demonstrated the efficacy of anti-VEGF in diabetic retinopathy and vein occlusion, the specific impact of anti-VEGF on retinal vascular dynamics, including vascular leakage, microaneurysms and ischemia, remains unclear.
| Take-home Point Quantitative assessment of perfusion and leakage dynamics in macular edema, whether due to diabetic retinopathy or retinal vein occlusion, can provide retina specialists with unique opportunities to understand the underlying pathology. Gaining a better understanding of these vascular changes and being able to obtain objective measurements may play an important role in monitoring disease progression and treatment response. |
The extent of peripheral retinal nonperfusion has been correlated with the severity of macular edema in diabetic retinopathy and vein occlusion. Michael A. Singer, MD, and colleagues found that eyes with increased peripheral nonperfusion had greater response to treatment with anti-VEGF, resulting in better visual acuity and decreased macular edema. Their study also suggested that eyes with macular edema are at higher risk of peripheral nonperfusion, underscoring the value of widefield angiography (Figure 1).19 While this study looked at qualitative changes in angiogram, few reports have demonstrated quantitative improvements with ischemia following anti-VEGF therapy.
Tools for Evaluating Angiographic Findings
Objective and quantitative measurement tools for angiographic features have been lacking. Current analysis is limited to the clinician’s subjective interpretation. Various investigators have described potential approaches to quantitative assessment of angiographic and widefield angiographic features. Colin Tan, MD, and colleagues in Singapore used stereographic projection software to correct for peripheral distortion and calculate areas of ischemia in anatomically correct physical units (mm2).20 Hossein Rabbani, MD, and
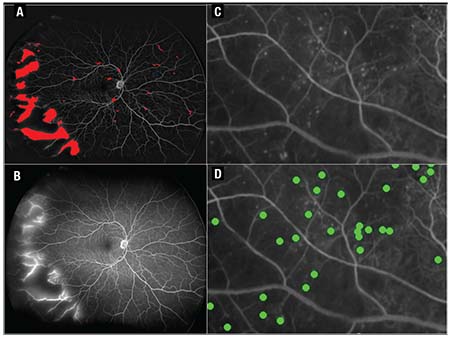 |
| Figure 2. Examples of quantification of leakage segmentation in red (A,B) and microaneurysms (C, D) shown in green in diabetic retinopathy. |
Quantitative assessment can provide unique opportunities for understanding underlying changes in retinal vascular dynamics. Moreover, objective measurements will likely play a pivotal role in tracking patients’ progression and response to treatment.
Studies are under way focusing on objective analysis of angiographic parameters in diabetic retinopathy. PERMEATE is an ongoing clinical trial investigating retinal vascular dynamics with a novel angiographic quantitative assessment tool in eyes with DME or retinal vein occlusion treated with intravitreal aflibercept. Preliminary six-month data showed study eyes treated with intravitreal aflibercept had improvement in visual acuity, decrease in the number of microaneurysms, less leakage and smaller areas of ischemia.24 Final results will be available soon. The REACT study is a recently completed study that is also investigating quantitative assessment of widefield angiography in eyes with treatment-resistant diabetic macular edema that were converted from bevacizumab to ranibizumab (ClinicalTrials.gov: NCT01982435).
Seeking Metrics for Following Diabetic Retinopathy
Because multiple therapeutics are undergoing evaluation as potential agents to treat diabetic retinopathy, clinicians need objective metrics to monitor treatment response. Ranibizumab recently received approval for diabetic retinopathy
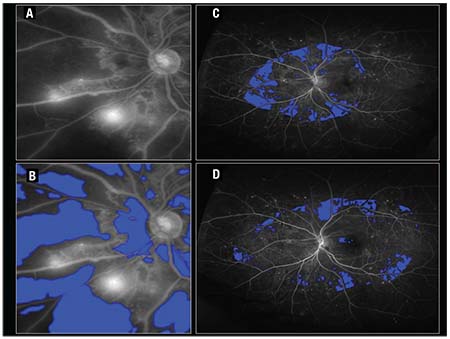 |
| Figure 3. Examples of ischemia segmentation seen in blue (A,B), at baseline (C) and at one year (D) in diabetic retinopathy. |
Another trial, PANORAMA, is assessing the efficacy of aflibercept for improvement of moderately severe to severe nonproliferative diabetic retinopathy (ClinicalTrials.gov: NCT02718326). Promising results from the TIME-2 study group demonstrated that AKB-9778 Tie2 activator (Aerpio Pharmaceuticals) combined with ranibizumab resulted in decreased diabetic macular edema compared to VEGF alone.26 Aerpio has reported that AKB-9778 is in a Phase IIb clinical trial to assess its role in stabilizing blood vessels to prevent vascular leakage and angiogenesis in diabetic retinopathy.
With the advent of intravitreal injections to treat diabetic retinopathy without macular edema, what are the metrics to follow these patients? What objective features can the clinician rely on? While optical coherence tomography (OCT) is indispensable to our decision making in managing macular edema, we need another tool to serve as a barometer in decision making for diabetic retinopathy and vein occlusion.
Although widespread availability of objective quantitative assessment of angiographic features is currently lacking, new and emerging technologies hold significant promise to enable objective measures of retinal vascular disease severity. Quantification and automated segmentation would allow for rapid and objective assessments of numerous disease features, including alterations in disease activity, pattern-based automated disease recognition or stratification, and individualized therapeutic decision making based on features and integrative pattern analysis. Additional research is needed to better understand the role for quantitative angiography for ongoing management of retinal vascular disease and for the potential impact on future clinical trial endpoints. RS
REFERENCES
1. Shweiki D, Itin A, Soffer D, Keshet E. Vascular endothelial growth factor induced by hypoxia may mediate hypoxia-initiated angiogenesis. Nature. 1992;359:843-845.
2. Thieme H, Aiello LP, Takagi H, Ferrara N, King GL. Comparative analysis of vascular endothelial growth factor receptors on retinal and aortic vascular endothelial cells. Diabetes. 1995;44:98-103.
3. Aiello LP, Avery RL, Arrigg PG, et al. Vascular endothelial growth factor in ocular fluid of patients with diabetic retinopathy and other retinal disorders. N Engl J Med. 1994;331:1480-1487.
4. Malecaze F, Clamens S, Simorre-Pinatel V, et al. Detection of vascular endothelial growth factor messenger RNA and vascular endothelial growth factor-like activity in proliferative diabetic retinopathy. Arch Ophthalmol. 1994;112:1476-1482.
5. Shima DT, Adamis AP, Ferrara N, et al. Hypoxic induction of endothelial cell growth factors in retinal cells: identification and characterization of vascular endothelial growth factor (VEGF) as the mitogen. Mol Med. 1995;1:182-193.
6. Nguyen QD, Shah SM, Van Anden E, Sung JU, Vitale S, Campochiaro PA. Supplemental oxygen improves diabetic macular edema: A pilot study. Invest Ophthalmol Vis Sci. 2004;45:617-624.
7. Nguyen QD, Tatlipinar S, Shah SM, et al. Vascular endothelial growth factor is a critical stimulus for diabetic macular edema. Am J Ophthalmol. 2006;142:961-969.
8. Campochiaro PA, Clark WL, Boyer DS, et al. Intravitreal aflibercept for macular edema following branch retinal vein occlusion: The 24-week results of the VIBRANT study. Ophthalmology. 2015;122:538-544.
9. Brown DM, Campochiaro PA, Bhisitkul RB, et al. Sustained benefits from ranibizumab for macular edema following branch retinal vein occlusion: 12-month outcomes of a phase III study. Ophthalmology. 2011;118:1594-1602.
10. Campochiaro PA, Brown DM, Awh CC, et al. Sustained benefits from ranibizumab for macular edema following central retinal vein occlusion: Twelve-month outcomes of a phase III study. Ophthalmology. 2011;118:2041-2049.
11. Scott IU, VanVeldhuisen PC, Ip MS, et al. Baseline factors associated with 6-month visual acuity and retinal thickness outcomes in patients with macular edema secondary to central retinal vein occlusion or hemiretinal vein occlusion: SCORE2 Study Report 4. JAMA Ophthalmol. 2017;135:639-649.
12. Tah V, Orlans HO, Hyer J, et al. Anti-VEGF therapy and the retina: An update. J Ophthalmol. 2015;2015:627674.
13. Rajappa M, Saxena P, Kaur J. Ocular angiogenesis: Mechanisms and recent advances in therapy. Adv Clin Chem. 2010;50:103-121.
14. Heier JS, Korobelnik JF, Brown DM, et al. Intravitreal aflibercept for diabetic macular edema: 148-week results from the VISTA and VIVID Studies. Ophthalmology. 2016;123:2376-2385.
15. Boyer D, Heier J, Brown DM, et al. Vascular endothelial growth factor Trap-Eye for macular edema secondary to central retinal vein occlusion: Six-month results of the phase 3 COPERNICUS study. Ophthalmology. 2012;119:1024-1032.
16. Brown DM, Schmidt-Erfurth U, Do DV, et al. Intravitreal Aflibercept for Diabetic Macular Edema: 100-Week Results From the VISTA and VIVID Studies. Ophthalmology. 2015;122:2044-2052.
17. Nguyen QD, Brown DM, Marcus DM, et al. Ranibizumab for diabetic macular edema: Results from 2 phase III randomized trials: RISE and RIDE. Ophthalmology. 2012;119:789-801.
18. Wells JA, Glassman AR, Ayala AR, et al. Aflibercept, bevacizumab, or ranibizumab for diabetic macular edema. N Engl J Med. 2015;372:1193-1203.
19. Singer M, Tan CS, Bell D, Sadda SR. Area of peripheral retinal nonperfusion and treatment response in branch and central retinal vein occlusion. Retina. 2014;34:1736-1742.
20. Tan CS, Chew MC, van Hemert J, Singer MA, Bell D, Sadda SR. Measuring the precise area of peripheral retinal non-perfusion using ultra-widefield imaging and its correlation with the ischaemic index. Br J Ophthalmol. 2016;100:235-239.
21. Rabbani H, Allingham MJ, Mettu PS, Cousins SW, Farsiu S. Fully automatic segmentation of fluorescein leakage in subjects with diabetic macular edema. Invest Ophthalmol Vis Sci. 2015;56:1482-1492.
22. Serlin Y, Tal G, Chassidim Y, et al. Novel fluorescein angiography-based computer-aided algorithm for assessment of retinal vessel permeability. PloS one. 2013;8:e61599.
23. Ehlers JP, Wang K, Vasanji A, Hu M, Srivastava SK. Automated quantitative characterisation of retinal vascular leakage and microaneurysms in ultra-widefield fluorescein angiography. Br J Ophthalmol. 2017;101:696-699.
24. Ehlers JP. Peripheral and macular reitnal vascular perfusion and leakage dynamics in diabetic macular edema and retinal venous occlusions during intravitreal aflibercept injection treatment for retinal edema: PERMEATE study. Paper presented at: Angiogenesis, Exudation and Degeneration 2017; February 11, 2017; Miami, FL.
25. Beaulieu WT, Bressler NM, Melia M, et al. Panretinal photocoagulation versus ranibizumab for proliferative diabetic retinopathy: patient-centered outcomes from a randomized clinical trial. Am J Ophthalmol. 2016;170:206-213.
26. Campochiaro PA, Khanani A, Singer M, et al. Enhanced benefit in diabetic macular edema from AKB-9778 Tie2 activation combined with vascular endothelial growth factor suppression. Ophthalmology. 2016;123:1722-1730.



