 |
 |
The discovery of anti-vascular endothelial growth factor and its effects on the pathogenesis of neovascular age-related macular degeneration drastically altered our treatment paradigm and the prognosis of patients.1 However, multiple studies suggest other pathways also contribute to pathologic angiogenesis.
In clinical practice, a substantial portion of patients still don’t respond to anti-VEGF monotherapy with bevacizumab or ranibizumab (Avastin and Lucentis, Genentech/Roche).4 Moreover, in the VIEW1 and VIEW2 studies, even though 95 percent of nAMD patients maintained their vision, only approximately 30 percent had an improvement of 15 or more letters in best-corrected visual acuity.2,3
The angiogenesis pathway
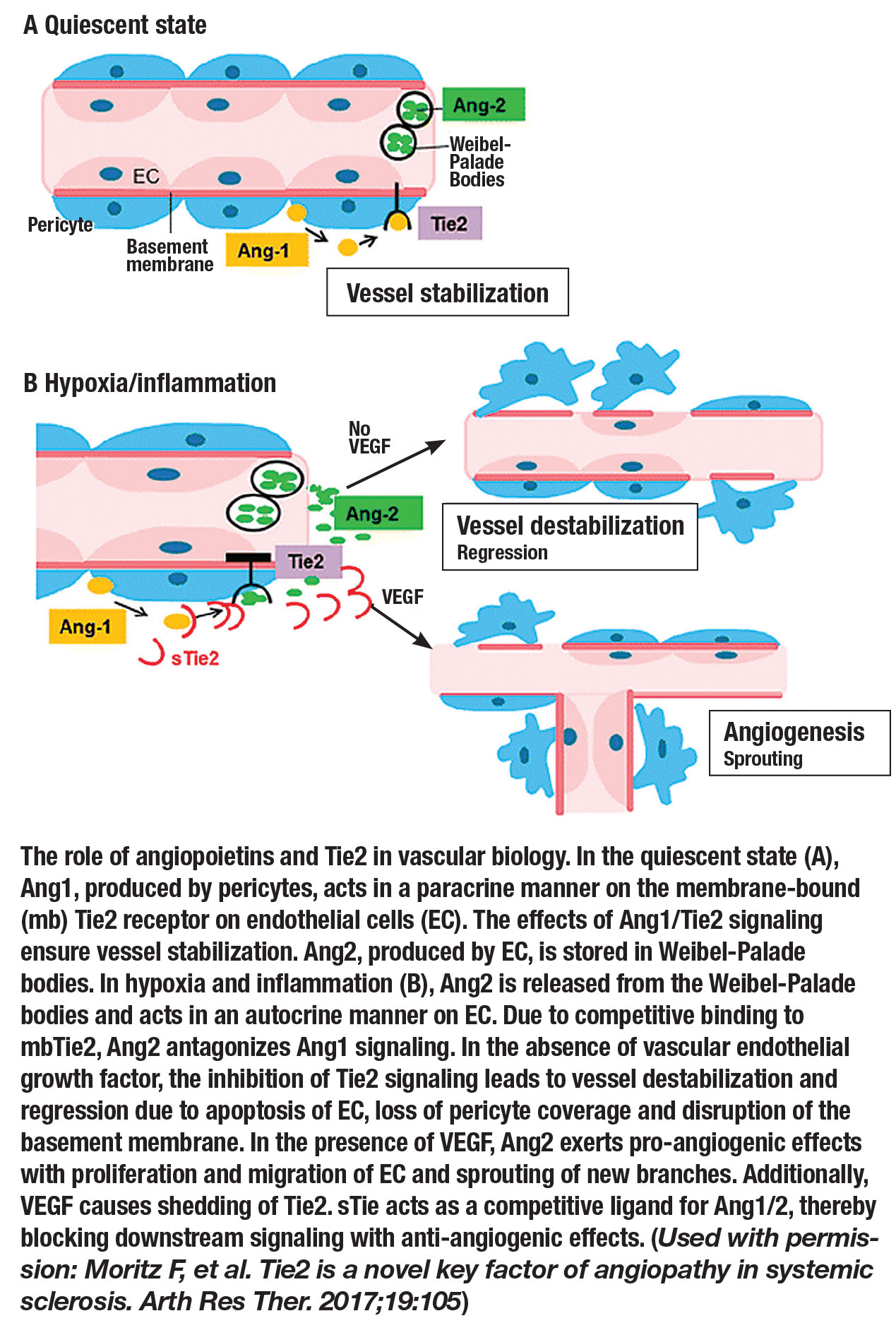
Angiopoietin-2 (Ang2) is a key molecule in the angiogenesis pathway and is directly involved in the Angiotensin1 (Ang1)/Tie2 signaling axis. Ang2 is produced almost exclusively by endothelial cells and functions as a vessel-destabilizing molecule through its competition with Ang1 and inhibition of Tie2.6 The central role of Ang2 in the angiogenesis cascade has led to intense exploration for the development of anti-
angiogenic drugs in ophthalmology and across other specialties.7-9
Similar to VEGF, Ang2 is upregulated by hypoxia, and ocular levels of both Ang2 and VEGF are elevated in eyes with nAMD. Additionally, multiple studies have demonstrated that VEGF and Ang2 are co-regulated in proangiogenic disease states and may also work together to induce pathological neovascularization and increased vascular permeability.10-14
These results seem to indicate that not only will inhibiting Ang2 provide therapeutic benefit in the treatment of nAMD, but combined inhibition of VEGF and Ang2 may produce a greater effect than either one individually.
Breakthrough Ang2 research
In 2016, researchers in Europe tested this theory of improved therapeutic benefit of combined inhibition of VEGF and Ang2.15 Utilizing a mouse model of aberrant retinal angiogenesis, they demonstrated that dual inhibition of VEGF-A and Ang2 significantly reduced vascular leakage when compared to not just controls, but also when compared to either Ang2 or VEGF inhibition alone.
The same team went on to design the first bispecific monoclonal antibody designed for the eye that binds to and inactivates VEGF-A and Ang2. RG7716 (now known as faricimab, Genentech/Roche) was first tested on non-human primates in a laser-induced CNV model. They compared faricimab to the anti-VEGF-A Fab fragment ranibizumab alone and
anti-Ang2 alone, and found that treatment with faricimab significantly reduced the amount of vessel leakage demonstrated on fluorescein angiography, and also reduced retinal architecture distortion.15
The Phase I trial of faricimab enrolled 24 patients with nAMD and refractory subfoveal choroidal neovascularization (leakage on FA or fluid on optical coherence tomography despite three or more intravitreal anti-VEGF treatments) who received single intravitreal doses of 0.5 mg, 1.5 mg, 3 mg and 6 mg of faricimab in a stepwise dose-escalation manner.16
Faricimab was tested in a multiple-dose phase where six patients received three treatments each of 3 mg and 6 mg. No dose-limiting toxicities were reported in either group; only one patient withdrew and one serious adverse event occurred (both of which were deemed to be unrelated to the study drug). Even in this small trial, both the stepwise dose-escalation group and the multiple-dose group had a median improvement in BCVA of at least 7 letters.
 |
Faricimab Phase II trials
 |
Two Phase II studies recently evaluated faricimab in nAMD. AVENUE17 is a proof-of-concept study that evaluated the effect of faricimab in patients with choroidal neovascularization secondary to AMD. AVENUE enrolled 273 patients in a multicenter, randomized, comparator-controlled parallel-group clinical trial to evaluate faricimab injections at four- and eight-week intervals (1.5 and 6.0 mg doses) vs. ranibizumab (0.5 mg) at four-week intervals in patients with nAMD. At 36 weeks, the study met its non-inferiority outcomes for vision and central retinal thickness.
STAIRWAY18 was a second Phase II study that evaluated the safety and efficacy of faricimab intravitreal injections at 16- (q16) and 12-week (q12) intervals in patients with nAMD compared to injections of ranibizumab every four weeks. Patients were treated with four loading doses of faricimab 6 mg at four-week intervals, and then were randomized into either q12 or q16 treatment intervals. At week 24 (12 weeks after the last of the four loading doses of faricimab), 65 percent (36/55) of patients treated with faricimab had no active disease.
In STAIRWAY, the following predefined criteria determined disease activity:
- increase in central subfield thickness on SD-OCT >50 µm vs. average over the prior two visits;
- increase in CST ≥75 µm vs. lowest over the prior two visits;
- decrease in BCVA due to nAMD of ≥5 letters vs. average over the prior two visits;
- decrease in BCVA due to nAMD of ≥10 letters vs. highest BCVA over the prior two visits;
- presence of new macular hemorrhage due to nAMD; or
- investigator opinion of significant nAMD disease activity at week 24 requiring immediate treatment.
 |
Additionally, initial improvements in BCVA (11.42, 10.08 and 9.59 letters gained in the q16 and q12 faricimab, and ranibizumab groups, respectively) were maintained in both the q16- and q12-week treatment groups.
CST was similar across all groups at 52 weeks (-122.5 µm, -138.5 µm and -129.9 µm in the q16- and q12-faricimab, and ranibizumab groups, respectively). No new safety signals were observed. The rates of ocular and systemic adverse events with faricimab were similar to ranibizumab.
Additional faricimab trials
Faricimab is being further evaluated for nAMD in two current Phase III trials funded by Genentech/Roche: TENAYA19 and LUCERNE.20 These identically designed, multicenter, randomized, double-masked, comparator-controlled studies aim to evaluate the efficacy, safety and durability of faricimab vs. aflibercept (Eylea, Regeneron Pharmaceuticals) in nAMD.
Between the two studies, almost 1,300 patients with nAMD will be randomized to receive either faricimab dosed at q16-week intervals (with the option of decreasing to q12 or q8 weeks), or aflibercept q8 weeks. The primary endpoint for both studies is change in BCVA at 48 weeks compared to baseline. Both studies, as of this writing, are fully recruited with an estimated primary completion date next year.
Faricimab is also being evaluated in the treatment of diabetic macular edema. Like the Phase III nAMD trials, YOSEMITE21 and RHINE22 are identical Phase III trials evaluating the efficacy of faricimab in the treatment of DME compared to aflibercept.
Nesvacumab
Regeneron is developing a second experimental monoclonal antibody, nesvacumab. Unlike faricimab, nesvacumab binds to and inhibits only Ang2. A Phase I, open-label, dose-escalation study evaluated the safety of intravitreal REGN910-3 (nesvacumab and intravitreal aflibercept injection [IAI]) in patients with either nAMD or diabetic macular edema.23 The two highest doses in this trial—REGN910-3 low-dose [3mg:2mg] and REGN910-3 high-dose [6mg:2mg]—were both well tolerated.
ONYX,24 the primary objective of which was to compare the efficacy of intravitreal REGN910-3 and IAI, was a Phase II, randomized, double-masked study in patients with nAMD. Results didn’t show sufficient differentiation between REGN910-3 and aflibercept alone. The aflibercept results in this study were consistent with findings from earlier clinical studies. Because of these findings, Regeneron announced the Phase III trial wouldn’t proceed after the Phase II results were released. In addition to ONYX, a similar study evaluating REGN910-3 in patients with DME, called RUBY,25 similarly showed insufficient differentiation from aflibercept alone.
Bottom line
Ang2 plays a clear role in angiogenesis through its inhibition of the Tie2 signaling pathway. Upregulation leads to pathological angiogenesis, both independently and through additive effects with VEGF. Animal models have demonstrated that inhibiting Ang2 leads to decreased vascular permeability, and dual inhibition of Ang2 and VEGF leads to significant reduction in vascular permeability greater than Ang2 or VEGF alone.
Faricimab, a bispecific monoclonal antibody to Ang2 and VEGF-A, has now gone through two Phase II trials, which have demonstrated noninferiority in both q16-and q12-week injections compared with q4-week ranibizumab when evaluating BCVA and CST.
On the other hand, nesvacumab, a monoclonal antibody that inhibits Ang2, in combination with aflibercept (REGN910-3) was found to be equivalent in efficacy to aflibercept alone. Phase III trials are under way comparing faricimab in q16 week intervals to aflibercept q8 weeks. RS
REFERENCES
1. Al-Zamil WM, Yassin SA. Recent developments in age-related macular degeneration: A review. Clin Interv Aging. 2017;12:1313-1330
2. Heier JS. VEGF trap-eye Phase III trial results. VIEW 1 results. Paper Presented at: Angiogenesis, Exudation, and Degeneration; February 12, 2011; Miami FL.
3. Schmidt-Erfurth U. VEGF trap-eye Phase III trial results. VIEW 2 results. Paper presented at: Angiogenesis, Exudation, and Degeneration; February 12, 2011; Miami FL.
4. Ng DS, Yip YW, Bakthavatsalam M, et al. Elevated angiopoietin 2 in aqueous of patients with neovascular age related macular degeneration correlates with disease severity at presentation. Sci Rep. 2017;7:45081. doi: 10.1038/srep45081.
5. Gale NW, Thurston G, Hackett SF, et al. Angiopoietin-2 is required for postnatal angiogenesis and lymphatic patterning, and only the latter role is rescued by angiopoietin-1. Dev Cell. 2002;3:411-423.
6. Fiedler U, Reiss Y, Scharpfenecker M, et al. Angiopoietin-2 sensitizes endothelial cells to TNF-alpha and has a crucial role in the induction of inflammation. Nat Med. 2006;12:235-239.
7. Koh YJ, Kim HZ, Hwang SI, et al. Double antiangiogenic protein, DAAP, targeting VEGF-A and angiopoietins in tumor angiogenesis, metastasis, and vascular leakage. Cancer Cell. 2010;18:171-184.
8. Huang H, Bhat A, Woodnutt G, Lappe R. Targeting the ANGPT-TIE2 pathway in malignancy. Nat Rev Cancer. 2010;10:575-585.
9. Mazzieri R, Pucci F, Moi D, et al. Targeting the ANG2/TIE2 axis inhibits tumor growth and metastasis by impairing angiogenesis and disabling rebounds of proangiogenic myeloid cells. Cancer Cell. 2011;19:512-526.
10. Shen J, Frye M, Lee BL, et al. Targeting VE-PTP activates TIE2 and stabilizes the ocular vasculature. J Clin Invest. 2014;124:4564-4576.
11. Lip PL, Chatterjee S, Caine GJ, et al. Plasma vascular endothelial growth factor, angiopoietin-2, and soluble angiopoietin receptor tie-2 in diabetic retinopathy: Effects of laser photocoagulation and angiotensin receptor blockade. Br J Ophthalmol. 2004;88:1543-1546.
12. Oshima Y, Oshima S, Nambu H, et al. Increased expression of VEGF in retinal pigmented epithelial cells is not sufficient to cause choroidal neovascularization. J Cell Physiol. 2004;201:393-400.
13. Oshima Y, Ishibashi T, Murata T, Tahara Y, Kiyohara Y, Kubota T. Prevalence of age related maculopathy in a representative Japanese population: The Hisayama study. Br J Ophthalmol. 2001;85:1153-1157.
14. Peters S, Cree IA, Alexander R, et al. Angiopoietin modulation of vascular endothelial growth factor: Effects on retinal endothelial cell permeability. Cytokine. 2007;40:144-150.
15. Regula JT, Lundh von Leithner P, Foxton R, et al. Targeting key angiogenic pathways with a bispecific CrossMAb optimized for neovascular eye diseases [published correction appears in EMBO Mol Med. 2019;11:e10666]. EMBO Mol Med. 2016;8:1265-1288.
16. Chakravarthy U, Bailey C, Brown D, et al. Phase I trial of anti-vascular endothelial growth factor/anti-angiopoietin 2 bispecific antibody RG7716 for neovascular age-related macular degeneration. Ophthalmol Retina. 2017;1:474-485.
17. Sahni J, Dugel PU, Patel SS, et al. Safety and efficacy of different doses and regimens of faricimab vs ranibizumab in neovascular age-related macular degeneration. The AVENUE phase 2 randomized clinical trial. JAMA Ophthalmolol. 2020;138:1-10.
18. Khanani AM, Patel SS, Ferrone PJ, et al. Efficacy of every four monthly and quarterly dosing of faricimab vs ranibizumab in neovascular age-related macular degeneration. The STAIRWAY phase 2 randomized clinical trial. JAMA Ophthalmolol. Published online July 30, 2020. doi:10.1001/jamaophthalmol.2020.2699
19. A Phase III, multicenter, randomized, double-masked, active comparator-controlled study to evaluate the efficacy and safety of faricimab in patients with neovascular age-related macular degeneration (TENAYA). ClinicalTrials.gov identifier: NCT03823287. Available at: https://clinicaltrials.gov/ct2/show/NCT03823287. Accessed June 23, 2020.
20. A Phase III, multicenter, randomized, double-masked, active comparator-controlled study to evaluate the efficacy and safety of faricimab in patients with neovascular age-related macular degeneration (LUCERNE). ClinicalTrials.gov identifier: NCT03823300. Available at: https://clinicaltrials.gov/ct2/show/NCT03823300. Accessed June 23, 2020
21. A Phase III, multicenter, randomized, double-masked, active comparator-controlled study to evaluate the efficacy and safety of faricimab in patients with diabetic macular edema (YOSEMITE). ClinicalTrials.gov identifier: NCT03622580. Available at: https://clinicaltrials.gov/ct2/show/NCT03622580. Accessed September 1, 2020.
22. A Phase III, multicenter, randomized, double-masked, active comparator-controlled study to evaluate the efficacy and safety of faricimab in patients with diabetic macular edema (RHINE). ClinicalTrials.gov identifier: NCT03622593. Available at: https://clinicaltrials.gov/ct2/show/NCT03622593. Accessed September 1, 2020.
23. Study of Intravitreal (IVT) REGN910-3 and IVT REGN910 in Patients With Either Neovascular ("Wet") Age Related Macular Degeneration (AMD) or Diabetic Macular Edema (DME). Clinical Trials.gov identifier: NCT01997164. Available at: https://clinicaltrials.gov/ct2/show/NCT01997164. Accessed September 1, 2020.
24. Anti-angiopoeitin 2 plus anti-vascular endothelial growth factor as a therapy for neovascular age related macular degeneration: Evaluation of a fixed combination intravitreal injection (ONYX). ClinicalTrials.gov identifier: NCT02713204. Available at: https://www.clinicaltrials.gov/ct2/show/NCT02713204. Accessed June 23, 2020.
25. Anti-vascular endothelial growth factor plus anti-angiopoietin 2 in fixed combination therapy: Evaluation for the treatment of diabetic macular edema (RUBY). ClinicalTrials.gov identifier: NCT02712008. Available at: https://clinicaltrials.gov/ct2/show/NCT02712008. Accessed June 23, 2020.



