Take-home Points
|
 |
|
Bios Ms. Narain is a medical student at George Washington University School of Medicine, Washington, D.C. Dr. Mishra is an vitreoretinal fellow at Byers Eye Institute, Stanford University, Palo Alto, California. Dr. Rahimy is a vitreoretinal specialist at Palo Alto Medical Foundation as well as adjunct clinical assistant professor in the Stanford Department of Ophthalmology. DISCLOSURES: Ms. Narain and Dr. Mishra have no relevant financial disclosures. Dr. Rahimy is a consultant to Regeneron Pharmaceuticals, Genentech/Roche, AbbVie/Allergan and Apellis. |
Concurrent with the strides achieved in treating diabetic eye disease with anti-VEGF therapy, systemic disease control has improved dramatically with emerging oral glucose-control therapies and, more recently, glucose monitoring technology. In diabetic retinopathy, some, but not all, of these advances have shown significantly improved outcomes in clinical trials, but in real-world studies, progression rates from nonproliferative to proliferative disease still remain high, similar to seminal historical studies.
These current real-world studies highlight the impact of current local therapies on retinopathy progression, although it’s important to recognize the limitations of big-data analyses before extrapolating conclusions regarding treatment and optimal timing of intervention.
Of the millions of people living with diabetes, one in three have some form of diabetic retinopathy, and more than one in 10 have vision-threatening DR.1 Understanding the rate of progression of non-proliferative DR without diabetic macular edema to proliferative DR (Figure 1) and baseline characteristics that predict progression, may inform optimal treatment strategies. Here, we report on take-homes from recent literature of DR progression in this age of anti-VEGF agents and next-generation diabetes management, and how that compares to historical evidence.
Early evidence of DR progression
DR progression has been well-documented in clinical trials for decades. In 1971, the Diabetic Retinopathy Study evaluated the effect of photocoagulation on DR.2 This multicenter, randomized clinical trial found that photocoagulation for eyes with proliferative disease with either argon or xenon arc reduced severe visual acuity loss (visual acuity 5/200 or worse) by approximately 50 percent compared with no treatment after five years of follow-up.
The Early Treatment Diabetic Retinopathy Study (ETDRS), another landmark study, assessed the effect of laser photocoagulation (scatter or focal) on DR and severe visual loss (Figure 2).3 In observed fellow eyes, 26.7 percent of eyes with baseline macular edema and less severe retinopathy developed high-risk PDR after five years, as did 61.3 percent of eyes with macular edema and more severe retinopathy.
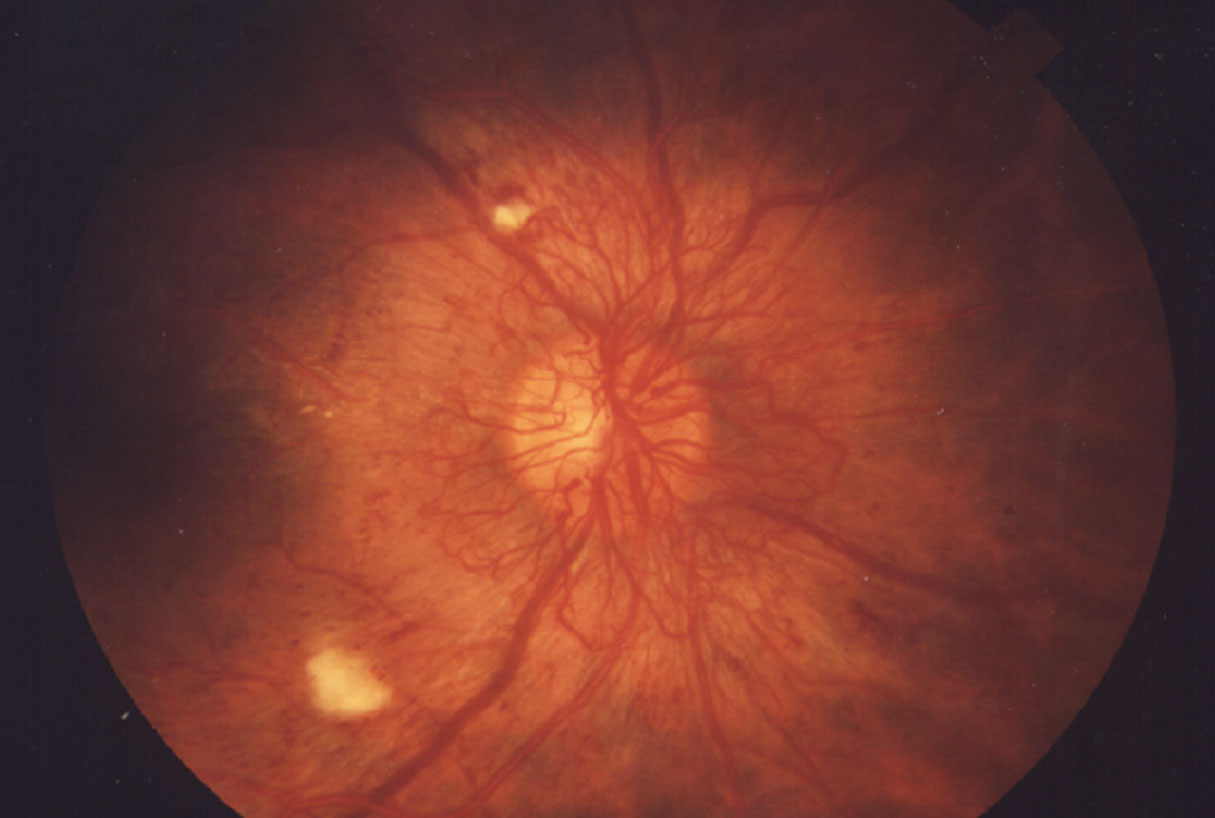 |
| Figure 1. A classic presentation of proliferative diabetic retinopathy showing abnormal vessel growth. (National Eye Institute) |
DR progression and newer diabetes drugs
More recent studies have also given us the opportunity to understand fellow-eye progression, particularly in more updated treatment schema. Significant advances in medicine and technology have expanded patients’ and retina specialists’ capacity to manage diabetes.
Newer diabetes drug classes that target distinct pathways, such as the sodium/glucose cotransporter member 2 (SGLT2) inhibitors, dipeptydil peptidase-4 (DPP-4) inhibitors and glucagon-like peptide-1 (GLP-1) agonists, offer alternatives for patients who struggle to control blood sugar levels with traditional treatments.
Continuous glucose monitors, such as Dexcom G6 (Dexcom) and FreeStyle Libre (Abbott), and diabetes coaching platforms for behavior modification, including Livongo and Virta Health, aim to smoothly integrate diabetes management into patients’ lives.
The effects of these advancements on real-time DR incidence, prevalence and progression rates aren’t yet understood. For example, semaglutide (Ozempic, Novo Nordisk), an increasingly popular GLP-1 agonist used to achieve optimal glycemic control, has been associated with worsening of DR in the pivotal SUSTAIN-6 clinical trial.4 In the study, retinopathy complication rates occurred in 3 percent (n=50) of the treatment group vs. 1.8 percent (n=29) of the control arm. The study authors postulated that this increased risk of progression was due to the rapidity and magnitude of blood glucose reduction from semaglutide.5
DR progression trends lower
A 2009 meta-analysis assessed the possibility of an effect of DR progression by more modern treatment strategies.6 The meta-analysis included observational studies reporting the progression of DR to PDR and/or progression to severe vision loss at four-, five- and 10-year intervals in two different time periods: from 1975 to 1985; and 1986 to 2008. Analysis revealed that nearly 60 percent of studies reported progression to PDR and 35 percent reported progression to severe vision loss (SVL).
When stratified for time period, studies reporting outcomes after four years showed that 19.5 percent of patients in 1975–1985 developed PDR compared with only 2.6 percent in 1986–2008. Also in the earlier interval, 9.7 percent of patients developed SVL compared with 3.2 percent in 1986–2008. These trends were consistent with those seen for five- and 10-year outcomes.
When stratified by baseline DR status, PDR developed in 6.3 percent of participants without DR at baseline in 1975–1985 compared with 2.6 percent in 1986–2008. Likewise, 2 percent developed SVL in 1975–1985 vs. none in 1986–2008.
For participants with DR at baseline, PDR developed in 39.7 percent in 1975–1985, and no studies reported progression to PDR during 1986–2008. Moreover, 17.5 percent of patients with DR at baseline progressed to SVL during 1975–1985, whereas 5.4 percent did so in 1986–2008.
The overall incidence of PDR and SVL observed in studies after 1985 were substantially lower than rates observed prior to 1985. These findings may be associated with the improvements in the overall care and management of diabetes, associated risk factors and earlier disease identification. One important limitation of this meta-analysis, however, is that baseline retinopathy was more severe in the earlier time period.
Anti-VEGF and DR progression
More recent clinical trials with fellow-eye data include the DRCR Retina Network Protocol W study, reported this year.7 This study explored whether anti-VEGF intervention for severe non-proliferative disease can limit progression to PDR. Eyes with moderate to severe NPDR treated with aflibercept (Eylea, Regeneron Pharmaceuticals) experienced a more than two-fold reduction in the incidence of PDR, while showing no difference in visual acuity between aflibercept-treated and sham groups at two years. One third of sham eyes developed PDR after two years. Based on baseline severity, 68 percent of patients with severe NPDR developed DME or PDR after two years.
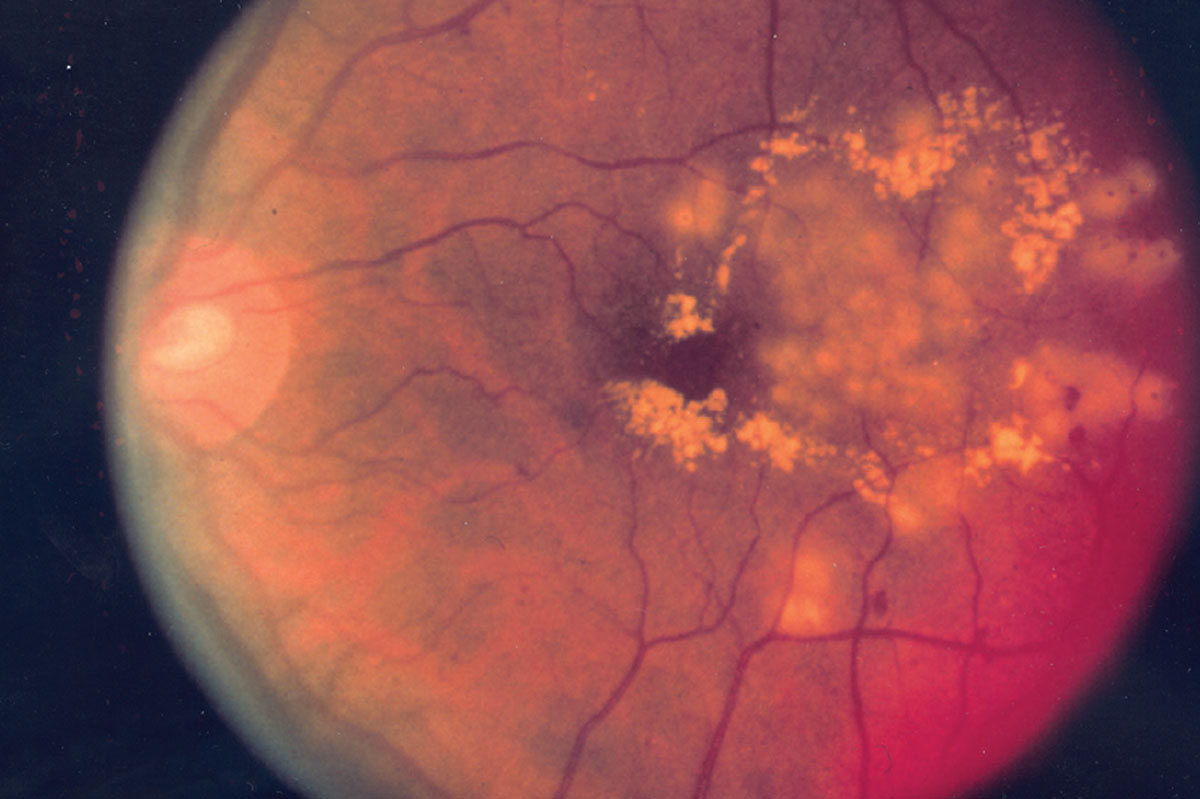 |
| Figure 2. Diabetic retinopathy post-laser treatment, which has shown variable efficacy in arresting progression to proliferative disease. (National Eye Institute) |
In the PANORAMA study, patients with diabetes and severe, treatment-naive NPDR were randomized into two different aflibercept regimens or sham.8 Results showed that at week 52, the event rate for a two-step or greater worsening in Diabetic Retinopathy Severity Scale was 1.6 percent in the aflibercept 2q 16-week group and 0 percent in the aflibercept 2q 8-week/pro re nata group compared with 11.9 percent in the controls.
The event rate for worsening at week 100 was also significantly lower in aflibercept patients, and those treated every eight weeks had lower rates than patients on 16-week regimens. At week 100, worsening was observed in 4.5 percent of the 16-week aflibercept patients and 2.4 percent of their eight-week/PRN counterparts compared with 20.2 percent in controls.
Risk of two-step worsening of DRSS
The risk of a two-step or greater worsening of DRSS level was significantly reduced by 89 percent at week 52 and 81 percent at week 100 in the aflibercept 2q 16-week group and by 100 percent at week 52 and 93 percent at week 100 in the 2q 8-week/PRN group vs. controls. These results indicate that aflibercept treatment of severe NPDR may reduce the progression of PDR in certain patients.
With regards to disease already in the proliferative stage, Protocol S demonstrated that 70 percent of eyes treated with panretinal photocoagulation and 65 percent of eyes treated with intravitreal ranibizumab (Lucentis, Genentech/Roche), remained at a PDR level (DRSS <61), with no improvement to NPDR level following two years of treatment.9
These findings parallel those of the CLARITY study, in which 90 percent of eyes treated with PRP and 78 percent treated with intravitreal aflibercept remained at the PDR level and with no advancement to the NPDR level following one year of treatment.10
But in the real world …
As valuable as clinical trials are in understanding DR progression, population-based studies afford the opportunity to understand real-world data and possibly negate the higher patient compliance rates seen in clinical trials.
One study by Geeta Lalwani, MD, and colleagues graded DR severity on the ETDRS DRSS in almost 42,000 eyes of 22,000 patients with diabetes, from 1999 to 2016 using the Inoveon database of patients with diabetes who participated in a retina screening program at primary clinical centers.11 The patients in the study were predominantly male (83 percent), while the ethnic distribution of the group was primarily Caucasian (50 percent) and Native American (41 percent).
The study found that 10 percent of all patients went on to develop a two-step DRSS worsening in five years. Patients with severe DR at baseline had higher incidence of two-step worsening. For patients with ETDRS DRSS scores of 43 to 53 and 47 to 53, almost 35 and 40 percent, respectively, had two-step DR worsening by year five.
 |
Retrospective EMR database study
A separate study utilized the Vestrum Health database, which included electronic medical records from 251 retina specialists across the United States, to describe the natural history of disease progression from nonproliferative to proliferative DR in patients without DME at baseline.1
This retrospective analysis included 135,324 patients, with data collected between January 1, 2013, and June 30, 2019. At 24 months, the cumulative incidence of conversion to PDR across anti-VEGF-naïve eyes with mild, moderate, and severe NPDR was 4.2, 11.1 and 28.6 percent, respectively. By 48 months, the figures had risen to 7.9, 20.9 and 46.8 percent.
Separately, the incidence of DME development at 24 months in the mild, moderate, and severe NPDR groups was 14.7, 33.7 and 44.1 percent. By 48 months, the proportion developing DME had grown to 27.1, 51.2 and 60.6 percent. Hence, the high proportion of patients with severe NPDR that progresses to PDR potentially introduces the opportunity for earlier anti-VEGF therapy rather than waiting for conversion to PDR.
Anti-VEGF and conversion rates
A separate arm of this study evaluated how anti-VEGF therapy may impact the progression of DR to proliferative disease. This part of the study included 10,142 eyes that received anti-VEGF and/or laser photocoagulation treatment prior to PDR conversion during the eligible study period.
The results demonstrated an increasing separation over time of conversion rates to PDR from mild to moderate to severe disease between patients who received anti-VEGF treatment and those who didn’t. The cumulative incidence of conversion to PDR ranged from 7.4 to 14.5 percent in patients with mild NPDR across treatment groups at 48 months, with the lowest incidence being in eyes that received prior anti-VEGF therapy (7.4 percent).
Similarly, for the moderate NPDR group, the conversion rate to PDR ranged from 11.6 to 20.9 percent at 48 months, with the lowest risk in the group receiving anti-VEGF therapy (11.6 percent), and the highest risk in the treatment-naïve group (20.9 percent).
Finally, in the severe NPDR group, progression to PDR ranged between 25.4 and 50.2 percent at 48 months. The highest risk of progression was in eyes with severe NPDR that had received prior laser but no anti-VEGF treatment (50.2 percent) and those that were entirely treatment-naïve (46.8 percent).
There was then a substantial separation from the remaining groups, where conversion rates to PDR was the lowest in anti-VEGF treated eyes: anti-VEGF plus laser (25.4 percent); and anti-VEGF monotherapy (27.5 percent).
Bottom line
Taken together, when left untreated, nearly half of all eyes with severe NPDR progressed to PDR within four years in routine clinical practice. Baseline NPDR severity was a strong predictor of progression to PDR, consistent with previously published studies.
These recent findings are nevertheless significant, because they suggest that even with the significant medical and technological advancements that have been incorporated in routine diabetes care, real-world progression rates are similar to historical clinical trials from a different time period.
Several important questions still exist regarding DR progression in the modern era. How will anti-VEGF therapy for severe NPDR affect real-world progression rates and retina specialist practice patterns? If further data show differences in how oral medications influence DR progression, will ophthalmologists play a role in guiding systemic management? Understanding newer systemic therapies may become even more crucial for today’s retina specialist. RS
REFERENCES
1. Khurana RN, Rahimy E, Boucher N. et al. Progression to proliferative diabetic retinopathy in nonproliferative diabetic retinopathy eyes without DME in the US. Presented at: Retina Society 2020 Virtual Annual Meeting; July 25, 2020.
2. Indications for photocoagulation treatment of diabetic retinopathy: Diabetic Retinopathy Study Report no. 14. The Diabetic Retinopathy Study Research Group. Int Ophthalmol Clin. 1987;27:239-253.
3. Early photocoagulation for diabetic retinopathy. ETDRS report number 9. Early Treatment Diabetic Retinopathy Study Research Group. Ophthalmology. 1991;98(5 Suppl):766-785.
4. Marso SP, Bain SC, Consoli A, et al. Semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med. 2016;375:1834-1844.
5. Sharma A, Parachuri N, Kumar N, et al. Semaglutide and the risk of diabetic retinopathy-current perspective. Eye (Lond). Published online August 9, 2021. doi: 10.1038/s41433-021-01741-5
6. Wong TY, Mwamburi M, Klein R, et al. Rates of progression in diabetic retinopathy during different time periods: a systematic review and meta-analysis. Diabetes Care. 2009;32:2307-2313.
7. Maturi RK, Glassman AR, Josic K, et al. Effect of intravitreous anti-vascular endothelial growth factor vs sham treatment for prevention of vision-threatening complications of diabetic retinopathy: The Protocol W randomized clinical trial. JAMA Ophthalmol. 2021;139:701-712.
8. Brown DM, Wykoff CC, Boyer D, et al. Evaluation of intravitreal aflibercept for the treatment of severe nonproliferative diabetic retinopathy: results from the PANORAMA randomized clinical trial. JAMA Ophthalmol. 2021;139:946–955.
9. Writing Committee for the Diabetic Retinopathy Clinical Research Network, Gross JG, Glassman AR, Jampol LM, et al. Panretinal photocoagulation vs. intravitreous ranibizumab for proliferative diabetic retinopathy: A randomized clinical trial. JAMA. 2015;314:2137-2146.
10. Sivaprasad S, Prevost AT, Vasconcelos JC, et al, for the CLARITY study group. Clinical efficacy of intravitreal aflibercept versus panretinal photocoagulation for best corrected visual acuity in patients with proliferative diabetic retinopathy at 52 weeks (CLARITY): A multicentre, single-blinded, randomised, controlled, Phase 2b, non-inferiority trial. Lancet. 2017;389:2193-2203.
11. Lalwani G, Wykoff CC, Briggs J, et al. DR progression analyses and its association with race and diabetes type in real-world patients with diabetes in the US. Invest Ophthalmol Vis Sci. 2021;62:1096.



