 |
Despite adequate wet AMD treatment that aims to achieve the clinical goals of maintaining a dry macula and avoiding recurrences, a significant proportion of patients note a slow, steady, and continuous decline in their visual function over time.
Not infrequently we find situations documenting a progressive loss of RPE that can clearly explain a patient’s difficulty reading (Figure 1). The situation becomes even more challenging if the patient requires frequent anti-VEGF treatments.
Anti-VEGF and RPE: No Consensus
Currently, no consensus exists on the potential effect of anti-VEGF therapy on the health of the RPE. Animal studies have indicated that anti-VEGF agents have a relevant effect on the choriocapillaris and its anatomic structure (e.g., loss of fenestration), and that drugs with higher affinity for vascular endothelial growth factor induce more such changes.1 Furthermore, if VEGF is neutralized experimentally, the RPE shows vacuoles and separation from the photoreceptor outer segments.2 These changes appear to be transient in cases where VEGF suppression is stopped.
Several clinical studies have found an effect of anti-VEGF inhibitors on the human choroid in different diseases, and it appears that a switch from ranibizumab (Lucentis, Roche/Genentech) to aflibercept (Eylea, Regeneron/Bayer) induces
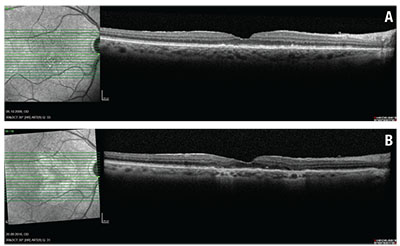 |
| Figure 1 .Optical coherence tomography B-scan through the fovea prior to any treatment shows no evidence of retinal pigment epithelium loss (A). The infrared images indicate one small area of RPE loss. OCT B-scan six years later (after 41 intravitreal ranibizumab injections) shows extended areas of hypertransmission with evidence of overlying photoreceptor loss indicating macular atrophy (B). The infrared image outlines the ring-shaped extent of the RPE loss around the fovea. |
We also started to realize that different types of RPE loss in neovascular AMD exist: loss directly associated with the neovascular lesion (Figure 2); and local RPE loss independent of choroidal neovascularization (Figure 1).
In the first case, differentiating the cause of the RPE loss is almost impossible. It may be due to the neovascular disease itself, VEGF inhibition or “successful” CNV regression. But consider the most worrisome situation for many of us: an AMD patient who requires retreatment at least every six weeks (close to continuous treatment) is switched to aflibercept with the aim of a longer treatment interval.
Our Retrospective Analysis
We performed our own retrospective analysis of such a neovascular AMD patient population (n=96), requiring retreatment at least every six weeks with ranibizumab or bevacizumab. Similar to other reports, we found that the subsequent switch to aflibercept leads to a modest but significant reduction in central retinal thickness, some effect on pigment
epithelial detachments (PEDs) and an increase in the treatment interval (5.6 to 6.9 weeks), but no improvement in vision.7 This raises the question if we are providing any benefit to the patient by using a drug with experimentally more effect on the choroid but with limited data on the rate of new RPE loss, location of RPE loss and association with lesion type or specific characteristics such as choroidal thickness or intraretinal fluid.8
This worry was exacerbated in our patient population (n=84) showing an accelerated reduction in choroidal thickness by optical coherence tomography following the switch to aflibercept.5 We were fortunate to have a full spectral-domain OCT dataset (Heidelberg Spectralis) on all 96 patients, allowing us to evaluate for OCT-defined RPE loss. We defined RPE loss/macular RPE atrophy—with no consensus on OCT-defined RPE loss at hand—as an area with hypertransmission and overlying photoreceptor band loss of at least 300 μm in diameter.
In addition
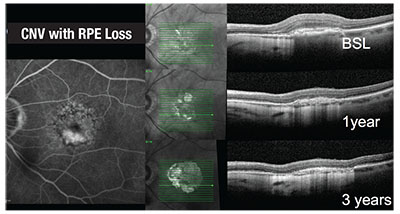 |
| Figure 2. This composite demonstrates progressive retinal pigment epithelium loss in the area of choroidal neovascularization in an eye with neovascular age-related macular degeneration undergoing a treat-and-extend regimen with aflibercept over three years. |
To avoid the confounding factor that large areas of RPE loss grow faster than small areas, we followed the suggestion of Philip Rosenfeld, MD, and co-authors to use a square-root transformation to compensate for this effect.9 In our population of frequently treated patients (median of 20 intravitreal injections over 24 months prior to switch), we found 17 of 96 eyes with evidence of RPE loss of at least 300 μm in diameter one year prior to switch.
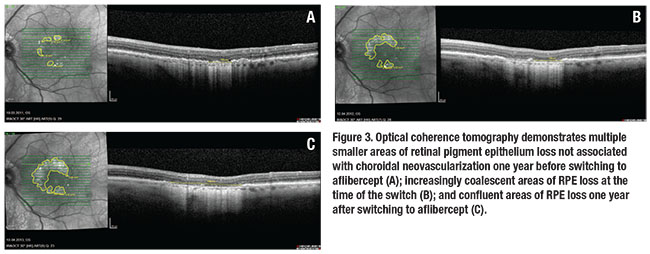
Based on the previous choroidal thickness analysis and selected cases (Figure 3), we expected an accelerated RPE loss with the switch to the VEGF inhibitor with higher affinity for VEGF. However, our study findings did not confirm our hypothesis.
 |
The area of RPE loss not associated with the CNV grew at a constant rate in the year before and the year after the switch.10 No acceleration on the progression of RPE loss could be detected up to three years after the switch. A potential explanation may be a minimal reduction in the number of median injections from 20 over the 24 months prior to the switch to 17 in the 24 months afterward.
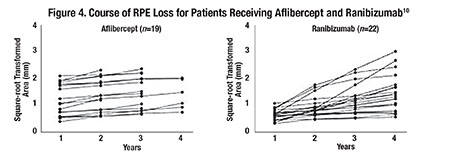
Outlining the area of RPE loss after square-root transformation of up to four years on aflibercept treatment and a comparable group on ranibizumab shows quite constant growth rates on the area of RPE loss (Figure 4). We actually found strong indicators for the linearity of the progression of RPE loss in both patient groups (mean R2 of 0.959).
Of course, we selected this patient population for non-CNV-related RPE loss, not for overall RPE loss. However, the patients did receive an adequate number of anti-VEGF injections associated with substantial VEGF suppression. So, it is reassuring that this presumably at-risk population did not show an accelerated RPE loss after the switch and over long-term follow-up.
On the other hand, this population may actually not be the one at risk for accelerated RPE loss, since the high need for anti-VEGF inhibition could indicate that the RPE produces adequate amounts of VEGF to protect the eye from the potentially adverse effects of VEGF suppression.
We also
 |
Our findings may help in decision-making regarding individual treatment strategies for patients with neovascular AMD that require frequent reinjections in order to maintain a dry retina. It appears that in this patient population, frequent and long-term anti-VEGF injections with ranibizumab or a switch to aflibercept do not accelerate the persistent rate of RPE loss most likely due to the underlying disease. Therefore, the highest risk for vision loss in this specific population remains under-treatment with anti-VEGF therapies.
Take-home Point
For patients with neovascular age-related macular degeneration that require frequent reinjections, under-treatment with anti-VEGF agents poses the highest risk for vision loss. No consensus exists on the potential effect of anti-VEGF drugs on the retinal pigment epithelium, although animal studies have indicated they affect the choriocapillaris and its anatomic structures, and that drugs with a higher affinity for VEGF induce more such changes. This article reports on a retrospective analysis that indicates frequent and long-term anti-VEGF injections with ranibizumab or a switch to aflibercept in this patient population do not accelerate the rate of RPE loss, and confirms previous reports that a switch to aflibercept may lead to a modest but significant reduction in central retinal thickness and have some effect on pigment epithelial detachments, but causes no improvement in vision. RS
REFERENCES
1. Julien S, Biesemeier A, Taubitz T, Schraermeyer U. Different effects of intravitreally injected ranibizumab and aflibercept on retinal and choroidal tissues of monkey eyes. Br J Ophthalmol. 2014;98:813-25.
2. Ford KM, Saint-Geniez M, Walshe T, et al. Expression and role of VEGF in the adult retinal pigment epithelium. Invest Ophthalmol Vis Sci. 2011 9;52:9478-9487.
3. Yiu G, Manjunath V, Chiu SJ, et al. Effect of anti-vascular endothelial growth factor therapy on choroidal thickness in diabetic macular edema. Am J Ophthalmol. 2014;158:745-751.
4. Kim JH, Lee TG, Chang YS, et al. Short-term choroidal thickness changes in patients treated with either ranibizumab or aflibercept: a comparative study. Br J Ophthalmol. 2016;100:1634-1639.
5. Mazaraki K, Fassnacht-Riederle H, Blum R, et al. Change in choroidal thickness after intravitreal aflibercept in pretreated and treatment-naive eyes for neovascular age-related macular degeneration. Br J Ophthalmol. 2015;99:1341-1344.
6. Chakravarthy U, Harding SP, Rogers CA et al. Alternative treatments to inhibit VEGF in age-related choroidal neovascularisation: 2-year findings of the IVAN randomised controlled trial. Lancet. 2013;382:1258-1267.
7. Pfau M, Fassnacht-Riederle HM, Freiberg FJ, et al. Switching therapy from ranibizumab and/or bevacizumab to aflibercept in neovascular age-related macular degeneration (AMD): One-year results. Klin Monbl Augenheilkd. 2016 ;233:945-590.
8. Kuroda Y, Yamashiro K, Ooto S, et al. Macular atrophy and macular morphology in aflibercept-treated neovascular age-related macular degeneration. Retina. 2017 Jul 4. Epub ahead of print
9. Feuer WJ, Yehoshua Z, Gregori G, et al. Square root transformation of geographic atrophy area measurements to eliminate dependence of growth rates on baseline lesion measurements: a reanalysis of age-related eye disease study report no. 26. JAMA Ophthalmol. 2013;131:110-111.
10. Wons J, Wirth MA, Graf N, et al. Comparison of progression rate of retinal pigment epithelium loss in patients with neovascular age-related macular degeneration treated with ranibizumab and aflibercept. J Ophthalmol. 2017 Feb 20. Epub ahead of print.



