Take-home Points
|
 |
|
Bios Dr. Wilkins is a clinical fellow in vitreoretinal surgery at New York Eye and Ear Infirmary of Mount Sinai, New York. Dr. Rosen is retina service chief and vice chair of research at New York Eye and Ear Infirmary. Disclosures: Drs. Wilkins and Rosen have no relevant disclosures. Preceyes and Mount Sinai Health System are in a strategic collaboration. Mount Sinai has an equity investment in Preceyes, and Preceyes has installed a surgical system at New York Eye and Ear Infirmary. |
The first-ever robotic-assisted surgery was reported by Yik San Kwoh, MD, and colleagues at Long Beach Memorial Medical Center in 1988, for which they used a robotic stereotactic biopsy platform used to aid in diagnosis of brain cancers.1
Since then, many fields of surgery have endeavored to use robotic technologies to aid in a variety of surgical procedures. Compared to other subspecialties, ophthalmologists have been relatively slow to adapt robotic-
assisted platforms, with the first-ever in-human study published in 2018.2
Ophthalmic surgery, particularly vitreoretinal surgery, demands extreme precision at the level of 40 µm or less, while an experienced surgeon manifests a tremor of roughly 100 µm, due to circulation and breathing.3,4 During static maneuvers this tremor can exceed 200 µm.3,4 Because of this human limit, there’s a need to address ways to improve manual dexterity and accuracy in vitreoretinal surgery. Robotics offer a potential avenue to do so.
Enhanced accuracy combined with haptic feedback for the surgeon may improve accuracy during delicate procedures such as fine macular work, retinal vessel cannulation and subretinal injections. The recent revolution of subretinal gene therapy, as exemplified by voretigene for RPE-65 biallelic mutation (Luxturna, Spark Therapeutics), will likely make robotic-assisted platforms even more valuable because of their high level of precision and accuracy.
We anticipate that these platforms will open up new avenues for treatment not previously possible with manual surgery alone. Here, we review the current
status of robotic-assisted vitreoretinal surgery and its future applications in clinical practice.
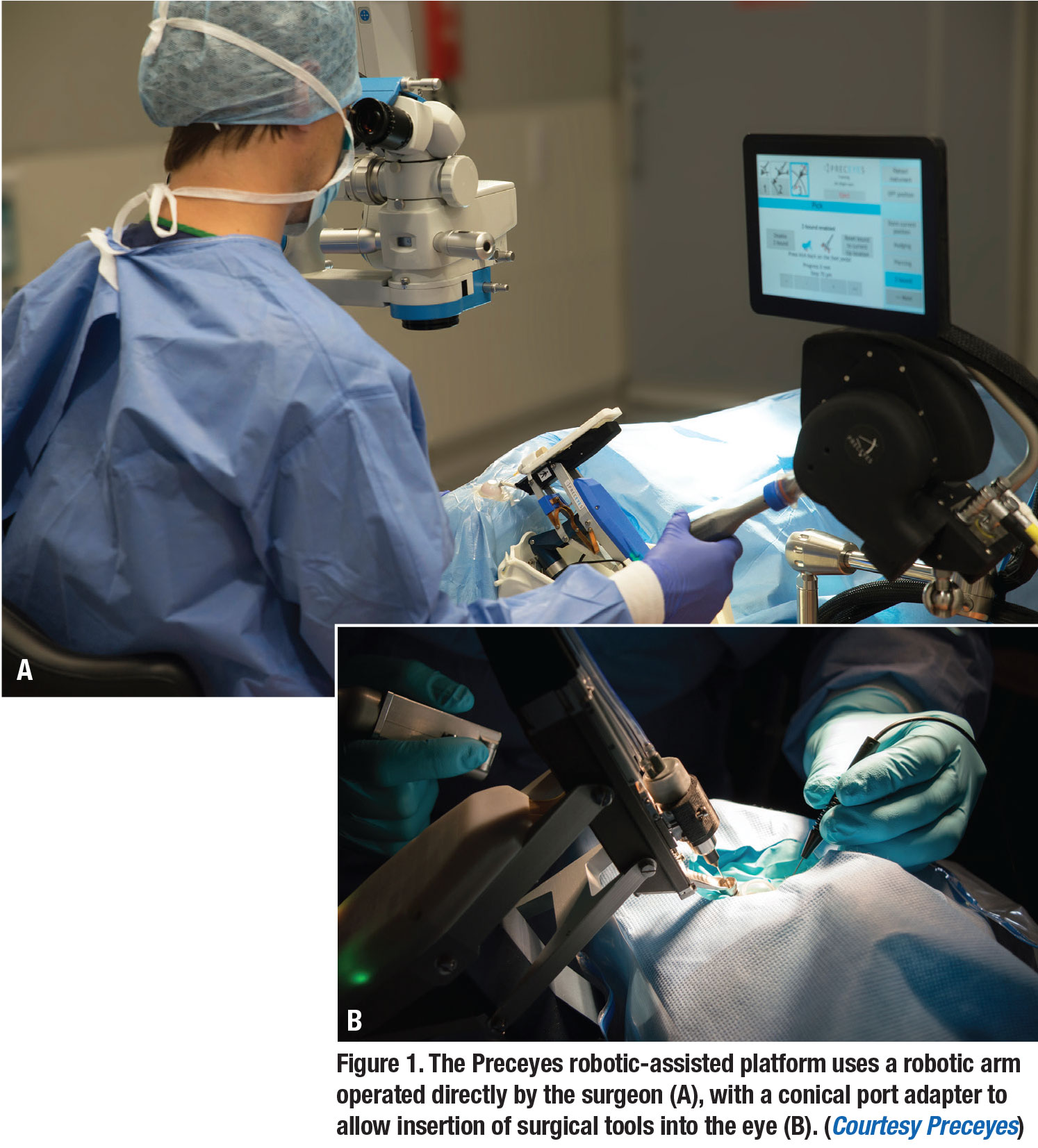
|
Categories of robotic-assisted platforms
The categories of robotic-assisted platforms in vitreoretinal surgery include hand-held assist devices and comanipulation and telemanipulation platforms. Hand-held devices, such as the Micron, are completely controlled. They’re handled by the surgeon and are designed with the main objective of minimizing surgeon tremor in order to augment surgical capabilities.
Comanipulative devices, such as the SteadyHand Eye robot from Johns Hopkins University, function by integrating the surgeon’s movements with limited robotic movements to optimize safety. The SteadyHand has five degrees of freedom and can mitigate unwanted surgeon movement by limiting rotational and X-Y-Z movement at a fixed incision point.5,6 The comanipulative abilities provide enhanced safety and precision of depth compared to the simpler hand-held assist platform; however, it’s larger and therefore may be more difficult to integrate into the operative workflow.
Telemanipulative platforms such as Preceyes (Figure 1) and the IRISS developed at UCLA offer the highest level of intraoperative precision due to the complete separation of the surgeon’s hand from the robotic manipulator.
The Preceyes robotic platform’s motion controller is operated by the surgeon, with the maneuvers translated to the robotic manipulator with a precision better than 20 µm. The IRISS platform has two robotic arms that allow the user’s wrist seven degrees of freedom.6 Though this type of platform is the largest, it has a head-mounted apparatus that attaches to most eye surgery beds and can be easily integrated into the operating room flow.
In the lab

|
Given their similarity to human eyes, porcine models have often been used in laboratory studies of new robotic-assisted platforms. In early studies, the IRISS platform demonstrated excellent efficacy with multiple vitreoretinal maneuvers, including core vitrectomy with posterior vitreous detachment induction, as well as cannulation of a retinal vein, without complications.
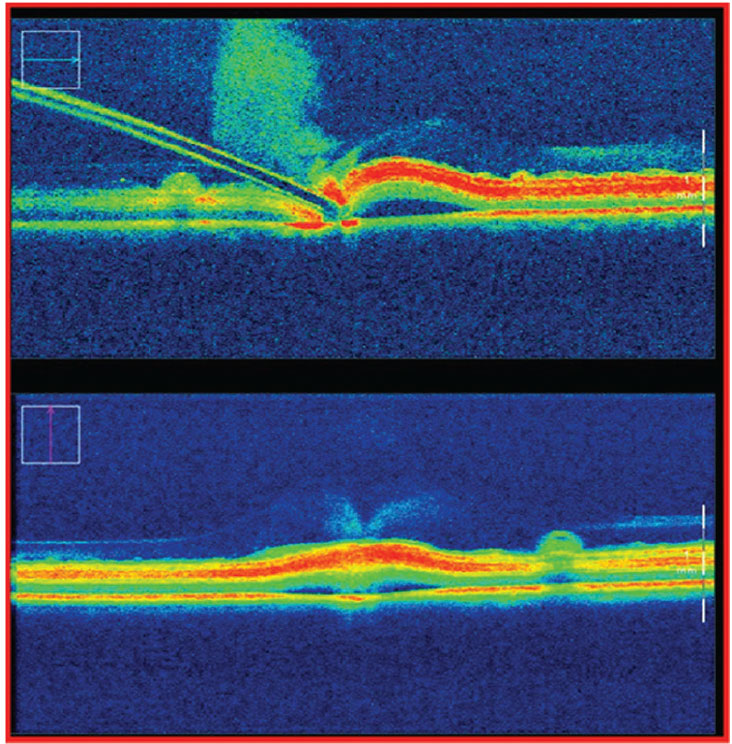
Other non-retinal procedures were also tested, including continuous curvilinear capsulorhexis and lens cortex removal.7 In ex-vivo porcine eyes, the Preceyes platform demonstrated excellent fidelity in subretinal injection procedures when measured with real-time intraoperative optical coherence tomography. It achieved successful bleb creation in 100 percent of eyes using robotic assistance compared with 40 percent using manual delivery. Bleb leakage occurred in all manual cases, compared to only 20 percent of robotic-assisted procedures7 (Figure 2).
Leakage is of extreme importance in subretinal therapy, which requires precise dosing of very expensive medications. Intraocular inflammation can also result from excessive escape of the agent into the vitreous.
This same robotic platform demonstrated reproducibility with cannulation of retinal veins when compared to manual attempts, with successful cannulation in 100 percent vs. 46 percent of porcine eyes in robotic-assisted and manual procedures, respectively.8 Repeated cannulation of the same entry point was also demonstrated to be reliable. These studies show that vitrectomy with cannulation of retinal vessels or subretinal drug delivery can be reliably achieved with reduced efflux of subretinal medication and reproducible cannulation of the microvasculature.
|
|
Figure 2. Subretinal bleb creation as measured by intraoperative optical coherence tomography during a manual procedure. Of note, on needle retraction, 100 percent of porcine eyes were shown to have efflux of subretinal injection, likely due to surgeon tremor. |
In-human experience
The report of the first-ever successful human clinical trial with robotic-assisted vitreoretinal surgery was published by Thomas Edwards and colleagues at Oxford University John Radcliffe Hospital in 2018.2 They used the Preceyes telemanipulation platform in patients undergoing 23-gauge macular surgery. They randomly assigned six patients to manual or robotic-
assisted macular hole repair under general anesthesia. Patients were enrolled for epiretinal membrane or internal limiting membrane peel based upon preoperative clinical appearance and OCT morphology.
An additional six patients with submacular hemorrhage were recruited for pars plana vitrectomy with subretinal tissue plasminogen activator (tPA) injection, also randomized to manual or robotic-assisted intervention. Each surgery used intraoperative OCT (Rescan 700, Carl Zeiss Meditec) for real-time assessment of residual membrane while peeling during macular hole repair, or to confirm delivery into the correct anatomic compartment during subretinal tPA injection.
For macular hole repair, times of surgery and flap creation were longer in the robotic group, but showed a trend toward reduced retinal microtrauma compared with manual surgery.2 While the difference wasn’t statistically significant, the number of subjects was small. The consensus of the surgeons involved was that with experience, the time to flap creation will drastically shorten, and that the trend toward reduced retinal trauma shows great promise in terms of improved clinical outcomes in the real world.
A second arm of the same study looked at subretinal macular hemorrhage patients who underwent subretinal tPA injection with air-fluid exchange under monitored anesthesia. All patients in both robotic and manual groups had successful subretinal bleb creation and postoperative displacement of hemorrhage. The investigators also successfully employed the “return to stored position” feature of the robotic system to return to a previously created microretinotomy for reinsertion of a microcatheter.2
|
Revolutionizing medication delivery
This feature of the technology promises to revolutionize medication delivery into microscopic spaces by eliminating undue trauma due to creation of false tracks, widening of the primary injection site, or injection into the wrong anatomic compartment with repetitive maneuvers. Reduction of tremor magnitude will also play a major role in the safety and efficacy of subretinal interventions, with less potential for unintended sub-RPE delivery or accidental retraction of the catheter leading to inadvertent efflux of medication. Additionally, repeated cannulation into the microvasculature will be accurate and reproducible, as laboratory research has demonstrated.8
Another randomized controlled trial for submacular hemorrhage demonstrated similar times for delivery of subretinal tPA between manual (6.7 minutes) and robotic (7 minutes) intervention, with no significant difference between postoperative displacement of hemorrhage or visual acuities.9 In this study, three separate training sessions were specified for surgeons and operating room staff, possibly contributing to the similar operating times between study groups. This suggests that efficiency in using the Preceyes system for performing delicate procedures improves with experience.
Potential barriers to implementation
|
A number of challenges remain before widespread adoption of robotic-assisted surgery can take place. Many experienced vitreoretinal surgeons will be hesitant at first to employ this nascent technology for cases they’re currently comfortable performing with traditional manual techniques. Studies of robotic surgery in other fields such as gynecology have shown that only about 6 percent of patients surveyed preferred robotic-
assisted surgery, mostly due to their perception of the speed of the procedure. Most patients had no preference (66 percent), while the rest preferred standard laparoscopic surgery (27 percent).10
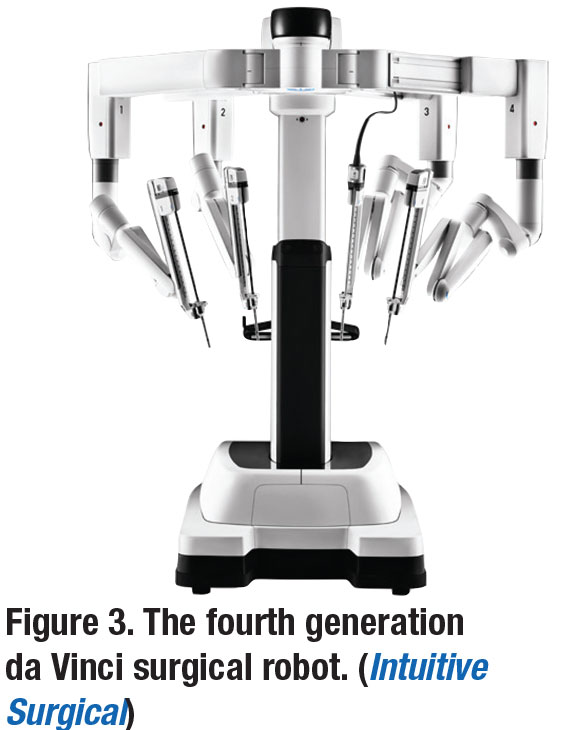
Cost may also be prohibitive for many surgical centers, considering that other established platforms such as da Vinci (Intuitive Surgical) cost up to $2 million and are far too large for ophthalmic use (Figure 3).
With decreasing reimbursements for many ophthalmic procedures, smaller surgical centers may not yield to pressure to integrate robotic platforms into the operating room. Additionally, training vitreoretinal surgeons to use these platforms will require an investment of time and resources that may not be available outside of tertiary referral centers.
Despite these challenges, it’s clear that vitreoretinal surgery is headed toward even more delicate microscopic procedures with increased demand for better patient safety metrics and outcomes, paving the way to increased acceptance and utilization of robotic-assisted platforms.
Future applications

|
In light of recent developments in subretinal therapeutics, combining robotic-assisted platforms and intraoperative image-guidance appears to be the logical next step toward achieving advanced surgical maneuvers beyond the capability of manual techniques.11
Intraoperative OCT is already established in manual surgery. Its uses range from assessing residual membrane or hyaloid in macular pucker surgery to providing immediate feedback during bleb creation in subretinal therapeutics.7 Telerobotic systems such as Preceyes provide “return-to-position” functioning, which, when combined with OCT, has the potential to enable surgeons to repeat image-guided subretinal injections while reducing the risk of inappropriate depth penetration or excessive medication efflux from the subretinal bleb.8,9,11
Telehealth is an additional area of interest for robotic platforms. Surgeons could perform complex procedures in distant locations without the need to travel. With an on-site, well-trained surgical team, this could bring expert surgeons to areas of need without the logistical difficulties and time demands of international outreach programs. It may ultimately be more cost-effective than the overall cost of mobilizing entire surgical teams to remote environments.
Bottom line

|
The precision and accuracy possible with the use of robotic-assisted vitreoretinal platforms will help vitreoretinal surgeons to potentially access areas previously inaccessible within the posterior segment. Combined with new intraoperative imaging capabilities, this technology is potentially transformative for vitreoretinal surgery.
Considering today’s rapidly evolving subretinal drug therapy developments, and the push for patient safety and operative efficiency in modern health care, surgeons will increasingly look to integrated surgical and imaging technologies to advance the field of vitreoretinal surgery. Robotic-assisted platforms are a promising advancement, offering stability and accuracy to extend the limits of human abilities and enhance our therapeutic options. RS
REFERENCES
1. Kwoh YS, Hou J, Jonckheere EA, Hayati S. A robot with improved absolute positioning accuracy for CT guided stereotactic brain surgery. IEEE Trans Biomed Eng. 1988;35:153-160.
2. Edwards TL, Xue K, Meenink HCM, et al. First-in-human study of the safety and viability of intraocular robotic surgery. Nat Biomed Eng. 2018;2:649-656.
3. Urias MG, Patel N, He C, et al. Artificial intelligence, robotics and eye surgery: Are we overfitted? Int J Retina Vitreous. 2019;5:52.
4. Roizenblatt M, Edwards TL, Gehlbach PL. Robot-assisted vitreoretinal surgery: Current perspectives. Robot Surg. 2018;5:1-11.
5. de Smet MD, Naus GJL, Faridpooya K, Mura M. Robotic-assisted surgery in ophthalmology. Curr Opin Ophthalmol. 2018;29:248-253.
6. Nuzzi R, Brusasco L. State of the art of robotic surgery related to vision: Brain and eye applications of newly available devices. Eye Brain. 2018;10:13-24
7. Ladha R, Smit J, Meenink T, et al. Defining parameters for robotic or manual reflux-free subretinal injections in an ex vivo animal model. Invest Ophthalmol Vis Sci. 2020;61:4485.
8. de Smet MD, Meenink TC, Janssens T, et al. Robotic assisted cannulation of occluded retinal veins. PLoS One. 2016;11:e0162037.
9. Kapetanovic JC, Xue K, Edwards TL, et al. First in human clinical trial of robot-assisted subretinal drug delivery under local anaesthesia. Invest Ophthalmol Vis Sci. 2019;60:2898.
10. Chu CM, Agrawal A, Mazloomdoost D, et al. Patients’ knowledge of and attitude toward robotic surgery for pelvic organ prolapse. Female Pelvic Med Reconstr Surg. 2019;25:279-283.
11. Ladha R, Meenink T, Smit J, et al. Advantages of robotic assistance over a manual approach in simulated subretinal injections and its relevance for gene therapy. Gene Ther. Published online May 17, 2021. https://doi.org/10.1038/s41434-021-00262-w.



