New Insights in Imaging
Take-home points
|
 |
|
Bios Ms. Pur is a medical student at Schulich School of Medicine and Dentistry, Western University, London, Ontario, and a junior scientist at the Octaine Imaging Lab. Ms. Sodhi is a medical student at the University of Cambridge, United Kingdom, and a senior scientist at the Octaine Imaging Lab. Dr. Choudhry is co-founder and medical director of Vitreous Retina Macula Specialists of Toronto and principal investigator at the Octane Imaging Lab. DISCLOSURES: Ms. Pur and Ms. Sodhi have no relevant disclosures. Dr. Choudhry disclosed relationships with Alcon, AbbVie, Apellis, Bayer, Carl Zeiss Meditec, Hoffman La Roche, Johnson & Johnson and Novartis. |
The retina consists of highly metabolically active tissue, and consequently it’s susceptible to mitochondria-related damage.1 Mitochondria play an essential role in maintaining cellular energy homeostasis, particularly at the level of the photoreceptors.2 Numerous studies have demonstrated that mitochondrial dysfunction is a key factor in the pathogenesis and progression of retinal diseases, such as diabetic retinopathy,3 central serous retinopathy,4 age-related macular degeneration,5,6 glaucoma,7,8 retinal dystrophies9 and optic nerve pathologies.10
Mitochondrial metabolic dysfunction renders the neural retina and retinal pigment epithelium vulnerable to oxidative stress, which can be measured using mitochondrial flavoprotein fluorescence (FPF), also called fluorescence lifetime imaging. This novel, noninvasive imaging modality functions as a marker of oxidative stress and mitochondrial dysfunction by quantifying the ratio of oxidized flavoproteins to reduced flavoproteins in mitochondria.11
Compared to spectral domain optical coherence tomography, the gold standard for diagnosis and monitoring of retinal diseases, which offers visualization of structural alternations, FPF provides information about subtle functional alternations that may precede structural changes.
Clinical utility of FPF imaging
Emerging studies highlight that FPF may be an indicator of retinal disease activity.12 Specifically, it may serve as a tool for early detection, classification and prognostication of retinal diseases, as well as evaluate a patient’s response to therapy. FPF was shown to detect and classify disease stages of AMD into early AMD, intermediate AMD, advanced geographic atrophy and advanced neovascular AMD based on mean differences in flavoprotein fluorescence heterogeneity.5,6 FPF has the potential to act as predictive marker of AMD disease activity, a promising idea given that little is known about a patient’s individual-level risk of AMD progression.
Moreover, FPF has been shown to positively correlate with the degree of DR3,12,13 and provide information regarding the therapeutic response to anti-VEGF that OCT may not capture.14 A pilot study of eight patients with DR and diabetic macular edema, in whom FPF was measured in the same eyes before and after anti-VEGF treatment, showed a significant negative correlation between improvement in best-corrected visual acuity and average FPF intensity values.14
Interestingly, the authors remarked that improvements in BCVA and increased FPF values (i.e., signaling improved mitochondrial function), but not OCT central macular thickness (CMT), occurred in anti-VEGF-treated patients with good vision and lower degrees of CMT.14 This suggests that FPF may evolve as a dynamic, early marker of treatment response, correlating with BCVA changes that precede those in OCT CMT.14
Potential in central serous retinopathy
Similar uses were reported in central serous retinopathy, specifically in a pilot study of three patients with CSR that reported detecting increased FPF average values as early as one week after symptom onset, compared with conventional FAF that detects changes several weeks after diseases onset.4
Furthermore, in a large cohort of 157 patients with inherited retinal dystrophies, mean FPF heterogeneity was found to correlate with FAF lesions, suggesting its relevance as a clinically relevant imaging marker.9
Other uses for FPF include disease detection and monitoring in optic nerve pathologies, open-angle glaucoma7 and expanding knowledge on neurodegenerative conditions such as Parkinson’s disease, Alzheimer’s and other forms of dementia.15,16
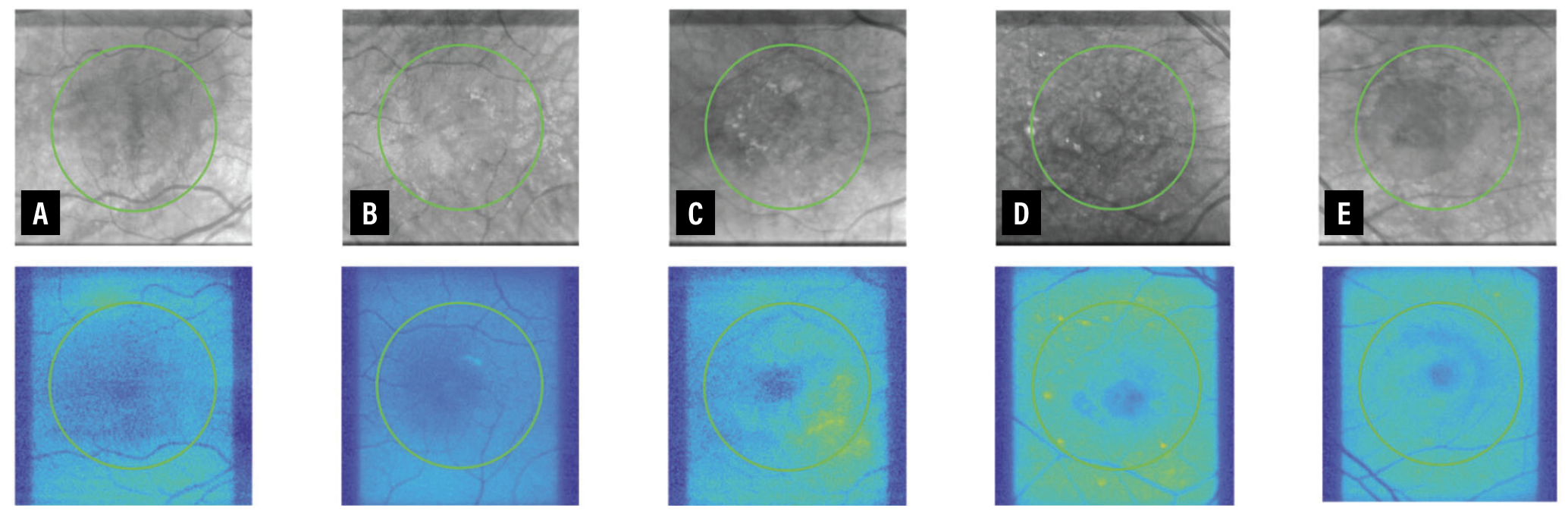 |
| A side-by-side comparison of intraretinal imaging (top row) and mitochondrial flavoprotein fluorescence (FPF, bottom row) for various disease states: A) healthy control; B) early age-related macular degeneration; C) intermediate AMD; D) advanced geographic atrophy; and E) advanced neovascular AMD. |
Advantages and disadvantages of FPF
FPF offers several benefits, such as noninvasively providing objective, functional information of early alternations in mitochondrial metabolism, which have been suggested to precede structural changes captured by OCT. However, the promise of this evolving technique must be considered in the context of several limitations.
First, no universal standards exist in the type of devices used, image processing parameters and healthy FPF reference values. For example, some authors have reported on FPF signal intensity while others reported on FPF signal heterogeneity. Furthermore, the choice of image processing parameters isn’t generally justified, rendering the methodology and device features a “black box.”
Our understanding is also evolving that patient factors such as age, gender, the presence of a cataract,13 corneal pathology17 and ocular or systemic comorbidities increase the variability in FPF signal and consequently decrease the reliability and generalization of reported findings.
We need larger studies that include well-defined populations, that clearly outline methodology and adjust for confounding factors following a standard protocol before we can widely use these devices in the clinic.
Future of FPF
FPF is emerging as a powerful yet little-known tool for the detection and classification of retinal disease, and for monitoring the therapeutic effects of treatment of retinal diseases. It adds a complementary way of interrogating and visualizing function of retinal processes.
Perhaps one of the most exciting avenues for FPF is its potential use in conjunction with therapeutics that target mitochondrial dysfunction in ocular diseases.18 Other future directions include its use along with artificial intelligence-based analyses of ocular biofluids and OCT imaging to provide care tailored to individual patients.19 RS
REFERENCES
1. Chen AX, Conti TF, Hom GL, et al. Functional imaging of mitochondria in retinal diseases using fl avoprotein fluorescence. Eye. 2021:35:74-92.
2. Kooragayala K, Gotoh N, Cogliati T, et al. Quantification of oxygen consumption in retina ex vivo demonstrates limited reserve capacity of photoreceptor mitochondria. Investig Ophthalmol Vis Sci. 2015;56:8428-8436.
3. Field MG, Elner VM, Puro DG, et al. Rapid, noninvasive detection of diabetes-induced retinal metabolic stress. Arch Ophthalmol. 2008;126:934-938.
4. Field MG, Elner VM, Park S, et al. Detection of retinal metabolic stress resulting from central serous retinopathy. Retina. 2009;29:1162-1166.
5. Muste JC, Russell MW, Chen AX, et al. Functional imaging of mitochondria in age-related macular degeneration using flavoprotein fluorescence. Ophthalmic Surg Lasers Imaging Retina. 2023;54:24-31.
6. Field MG, Comer GM, Kawaji T, Petty HR, Elner VM. Noninvasive imaging of mitochondrial dysfunction in dry age-related macular degeneration. Ophthalmic Surg Lasers Imaging. 2012;43:358-365.
7. Zhou DB, Castanos MV., Geyman L, et al. Mitochondrial dysfunction in primary open-angle glaucoma characterized by flavoprotein fluorescence at the optic nerve head. Ophthalmol Glaucoma. 2022;5:413-420.
8. Ritch R, Suwan Y, Rosen RB, De Moraes CG. A randomized, double-masked, placebo-controlled trial of the efficacy of a novel neuroprotective combination for reversing mitochondrial dysfunction in glaucoma. Poster presented at the American Glaucoma Society 2018 annual meeting; New York; March 2, 2018.
9. Russell MW, Muste JC, Seth K, et al. Functional imaging of mitochondria in genetically confirmed retinal dystrophies using flavoprotein fluorescence. Ophthalmic Genet. 2022;43:834-840.
10. Elner VM, Park S, Cornblath W, Hackel R, Petty HR. Flavoprotein autofluorescence detection of early ocular dysfunction. Arch Ophthalmol. 2008;126:259-260.
11. Ning X, Baoyu Q, Yuzhen L, Shuli S, Reed E, Li QQ. Neuro-optic cell apoptosis and microangiopathy in KKAY mouse retina. Int J Mol Med. 2004;13:87-92.
12. Elner SG, Elner VM, Field MG, Park S, Heckenlively JR, Petty HR. Retinal flavoprotein autofluorescence as a measure of retinal health. Trans Am Ophthalmol Soc. 2008;106:215-222.
13. Chen AX, Conti TF, Hom GL, et al. Functional imaging of mitochondria in retinal diseases using flavoprotein fluorescence. Eye. 2021;35:74-92.
14. Andrade Romo JS, Lynch G, Liu K, et al. Flavoprotein fluorescence correlation with visual acuity response in patients receiving anti-VEGF injection for diabetic macular edema. Oxid Med Cell Longev. 2018:3567306. doi:10.1155/2018/3567306
15. Blazes M, Lee CS. Understanding the brain through aging eyes. Adv Geriatr Med Res. Published online March 1, 2021. doi: 10.20900/agmr20210008.
16. Barlow CH, Harden WR 3rd, Harken AH, et al. Fluorescence mapping of mitochondrial redox changes in heart and brain. Crit Care Med. 1979;7:402-406.
17. Rovati L, Docchio F. Autofluorescence methods in ophthalmology. J Biomed Opt. 2004;9:9.
18. Huang C-P, Lin Y-W, Huang Y-C, Tsai F-J. Mitochondrial dysfunction as a novel target for neuroprotective nutraceuticals in ocular diseases. Nutrients. 2020;12:1950.
19. Pur DR, Krance SH, Pucchio A, Miranda RN, Felfeli T. Current uses of artificial intelligence in the analysis of biofluid markers involved in corneal and ocular surface diseases: A systematic review. Eye. Published online November 15, 2022. doi:10.1038/s41433-022-02307-9.



