Take-home points
|
 |
|
Bios Dr. Thura is an ocular oncology fellow at Bascom Palmer Eye Institute, University of Miami. |
The development and widespread use of new approaches to treat cancer, including immunotherapy and targeted therapies, has altered the landscape of cancer treatment to provide novel ways to improve tumor control and patient survival. Targeted cancer therapies inhibit the growth and spread of cancer by impeding the action of specific molecules that promote carcinogenesis, while immunotherapy helps the immune system better identify, attack and kill cancer cells. These approaches differ from the mechanism of traditional chemotherapeutic agents that kill all rapidly dividing cells.1,2
As clinicians embrace and employ these novel immunotherapies, targeted therapies and chemotherapy agents, distinct potential adverse events emerge. Ocular side effects of these medications can range from extremely rare to very common, and from mild and reversible to severe and irreversible, depending on the drug.
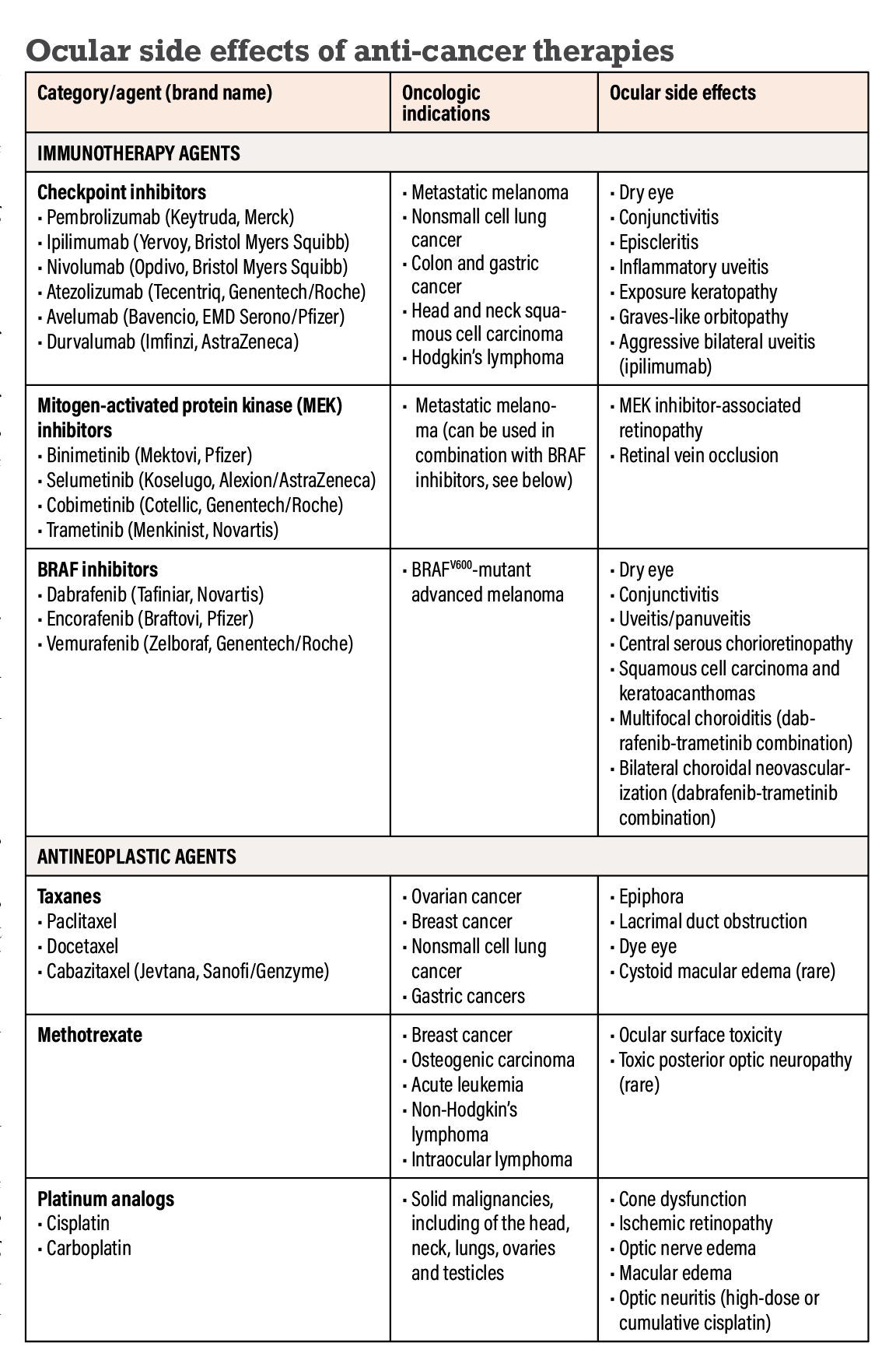
Here, we’ll outline a few of the emerging immunotherapies, chemotherapies and targeted therapies and discuss some of their known potential manifestations of ocular toxicity (Table below).
IMMUNOTHERAPY AGENTS
Checkpoint inhibitors
The mechanism of immune checkpoint inhibitor medications is to act on proteins that regulate T-cell activity. Inhibition of these proteins allows T-cells to become activated, resulting in an immune response to help fight metastatic cancers.
The proteins inhibited by this class include cytotoxic T-lymphocyte antigen-4 (CTLA-4), programmed death protein 1 (PD-1) and programmed death ligand (PD-L1).2,3 Some of the checkpoint inhibitors approved by the Food and Drug Administration include pembrolizumab (Keytruda, Merck), ipilimumab (Yervoy, Bristol Myers Squibb), nivolumab (Opdivo, Bristol Myers Squibb), atezolizumab (Tecentriq, Genentech/Roche), avelumab (Bavencio, EMD Serono/Pfizer) and durvalumab (Imfinzi, AstraZeneca). These medications are most commonly used to treat metastatic melanoma, nonsmall cell lung cancer, colon cancer, gastric cancer, head and neck squamous cell carcinoma and Hodgkin’s lymphoma.3,4
• Ocular side effects. The ocular side effects are generally immune-related and have been reported to occur in around 1 percent of patients weeks to months after treatment starts. The most common are dry eye, conjunctivitis, episcleritis, inflammatory uveitis, exposure keratopathy, and orbital inflammation (Graves-like orbitopathy), particularly noted with the CTLA-4 inhibitor ipilimumab.5
Ipilimumab has also been rarely associated with aggressive bilateral uveitis similar to the exudative retinal detachments seen in Vogt-Koyanagi-Harada syndrome.6
With anti-PD-1 and PD-L1 agents such as pembrolizumab and nivolumab, uveitis is a rare event but requires attention for appropriate management when it does occur (Figure 1 below).4,7 Uveitis has been noted at a rate of 0.3 to 6 percent in patients treated with checkpoint inhibitors,8 and it sometimes requires topical or oral corticosteroids to prevent permanent visual loss.
Concerns for potential autoimmune disease, such as myasthenia gravis and thyroid disease, and worsening of paraneoplastic syndromes should be considered while patients are on checkpoint inhibitor therapy.9
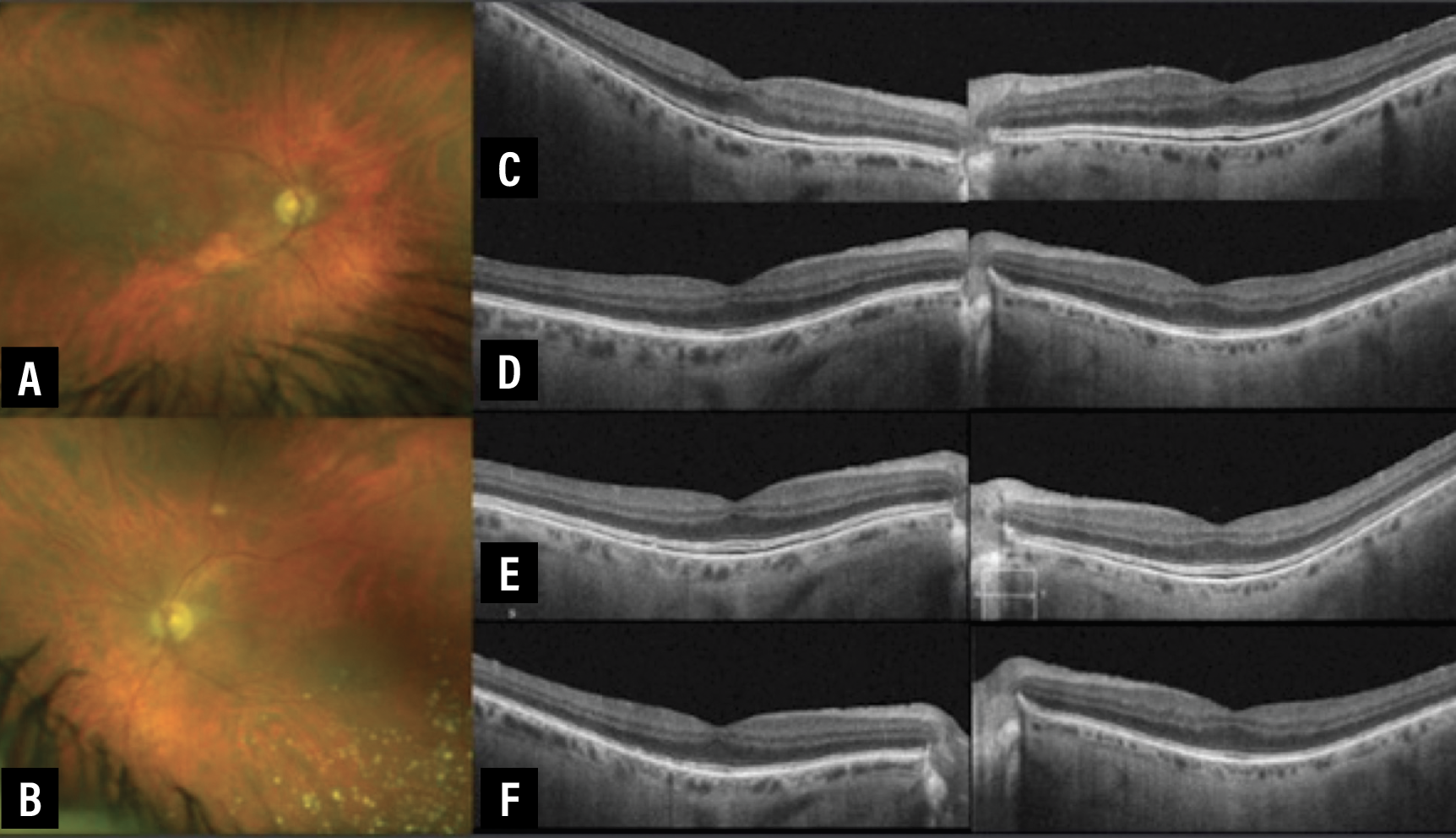 |
| Figure 1. This asymptomatic 75-year-old male had a metastatic cutaneous melanoma treated with the MEK inhibitor binimetinib. He started on the BRAF inhibitor encorafenib two months earlier. A and B) Fundus photographs of the right and left eyes in January 2020. C) Optical coherence tomography shows subretinal fluid in both the right (left image) and the left (right image) eyes in January 2020. Best-corrected visual acuity at this point was 20/30 OD and 20/25 OS. D) OCT one month later with BCVA OD and OS 20/30. E) OCT in May 2020 with BCVA OD and OS 20/25. F) OCT in November 2020 with BCVA OD and OS 20/30. |
MEK inhibitors
Mitogen-activated protein kinase inhibitors target the MAP kinase pathway, aberrations in which are involved in some cancers, such as metastatic melanoma.10 The combination of BRAF (discussed later) and MEK inhibitor therapy was created for treatment of patients with unresectable or metastatic BRAF-mutated melanoma.
MEK inhibitors include the medications binimetinib (Mektovi, Pfizer), selumetinib (Koselugo, Alexion/AstraZeneca), cobimetinib (Cotellic, Genentech/Roche) and trametinib (Mekinist, Novartis), which target different stages of the signaling pathway.
• Ocular side effects. MEK inhibitor-associated retinopathy (MEKAR) tends to occur early in the course of treatment, usually within days to weeks of starting therapy (Figure 1). MEKAR is often transient and may resolve without permanent effects after discontinuation of treatment. The incidence of MEKAR has varied in the literature from 5 to 75 percent, due to the range of clinical presentations such as bilateral serous retinal detachments, macular edema and outer retinal layer disruption.9,11
Researchers at Memorial Sloan Kettering Cancer Center in New York assessed 25 patients receiving MEK-inhibitor treatment, 92 percent of whom developed bilateral subretinal fluid foci. They reported that MEKAR is often characterized by bilateral and multifocal pockets of subretinal fluid without increased choroidal thickness, which helps to differentiate it from central serous chorioretinopathy (CSCR).12
Retinal vein occlusion, although rare, is a potential side effect that has been reported in clinical trials of some MEK inhibitors, namely trametinib.13 Vascular disease risk factors or glaucoma could predispose a patient to this type of event, which warrants evaluation by a retinal specialist.
 |
| Click image to enlarge. |
BRAF inhibitors
BRAF inhibitors—BRAF stands for B-Raf proto-oncogene—including dabrafenib (Tafinlar, Novartis), encorafenib (Braftovi, Pfizer) and vemurafenib (Zelboraf, Genentech/Roche), which also act along the MAP kinase pathway. They’re approved for the treatment of advanced cutaneous melanoma.14
• Ocular adverse events. OAEs that have been reported with BRAF treatment are dry eyes, conjunctivitis, uveitis, CSCR and various eyelid lesions ranging from rashes to keratoacanthomas and squamous cell carcinoma.2,15–17 Prompt biopsy and excision of squamoproliferative eyelid lesions, which can occur with vemurafenib or dabrafenib, is recommended, but discontinuation of therapy isn’t usually necessary.
The time from treatment initiation to detection of adverse events has been reported to range from a few weeks to a few months. In a large retrospective review of 568 patients who were treated with vemurafenib, 22 percent developed ocular events, the most common being uveitis, conjunctivitis and dry eye.18
Combination BRAF-MEKi therapy
Oncologic studies have shown that combination BRAF and MEK inhibitor therapy has additive antitumor effects, but the possibility of increased ocular toxicity in this setting remains controversial.
• Ocular adverse events. An observational pharmacovigilance study to assess OAEs with BRAF and MEK inhibitors noted a significant association between combination therapy and all types of uveitis, as well as serous retinal detachment.14
The combination of dabrafenib and trametinib has been reported to cause multifocal choroiditis and bilateral choroidal neovascularization.17 Severe panuveitis may warrant discontinuing immunotherapy if local therapy or steroids don’t control the inflammation.
ANTINEOPLASTIC AGENTS
Taxanes
The taxanes inhibit cancer cell proliferation through suppression of microtubule dynamics by stabilization leading to mitotic arrest.19 This class includes paclitaxel and docetaxel, both of which have multiple brand names, and cabazitaxel (Jevtana, Sanofi/Genzyme). They’re used for the treatment of ovarian cancer, breast cancer, nonsmall cell lung cancer, gastric cancers and, with cabazitaxel, advanced prostate cancer.
• Ocular adverse events. The most commonly reported OAEs include epiphora, lacrimal duct obstruction and ocular surface dryness, with the frequency of tearing noted to be as high as 88 percent while on treatment.20 The length of treatment and cumulative dosing have been shown to relate to disease severity; the severe end of the spectrum resulting in progressive nasolacrimal duct obstruction that may warrant surgery.
A handful of case reports of cystoid macular edema have been published, although its frequency isn’t well understood.1,21 The macular edema has been described as intraretinal cystoid spaces with dome-shaped foveal configuration, nonleaking on fluorescein angiography, with spontaneous resolution after withdrawal of the taxane drug.21
 |
|
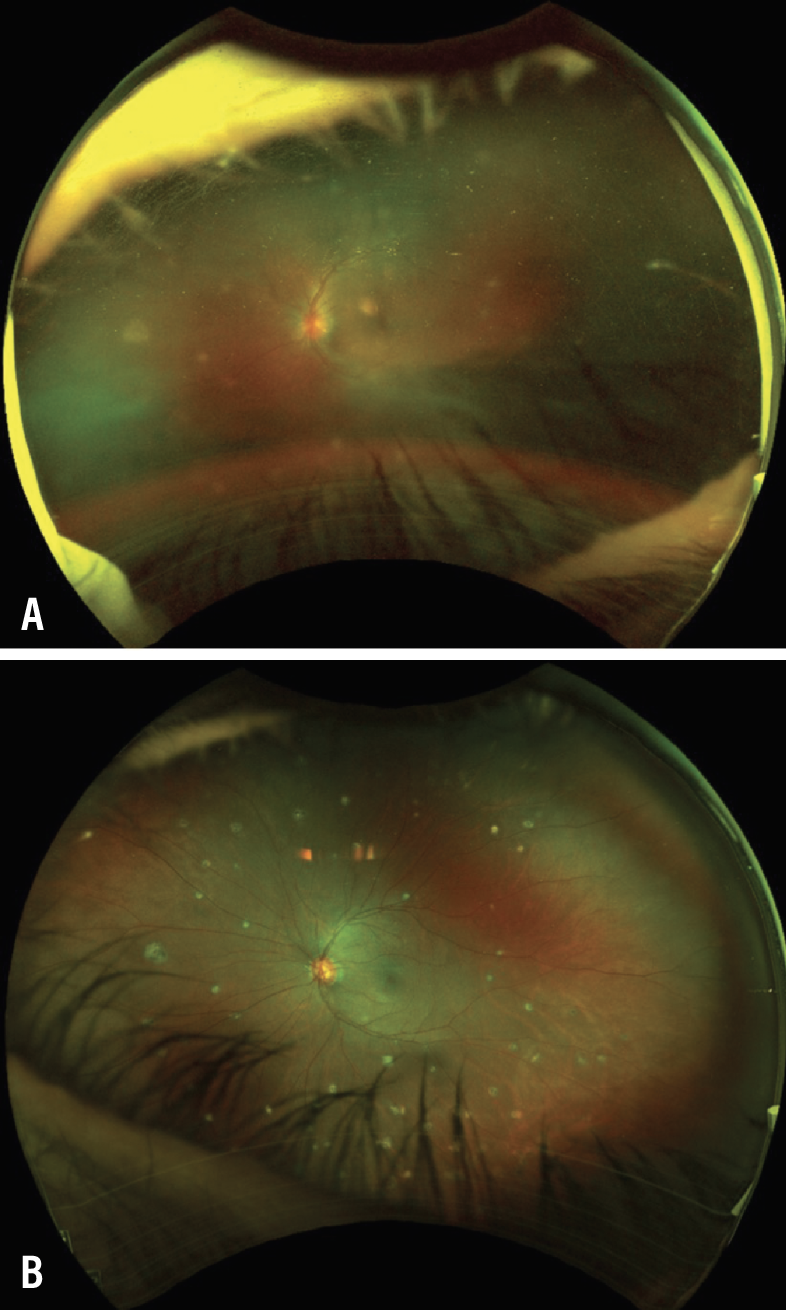
Figure 2. A) Fundus photograph of the left eye of a 35-year-old patient who developed bilateral panuveitis while on pembrolizumab, a PD-1 checkpoint inhibitor, for treatment of Hodgkin's lymphoma. Vitritis and white peripheral punctate lesions and vasculitis are notable. Best-corrected visual acuity is 20/40. B) Months after a sub-Tenon's Kenalog injection in the left eye, the vitritis improved significantly and BCVA improved to 20/20. The patient had also been taken off pembrolizumab several months earlier. The scattered peripheral lesions remain. |
Methotrexate
An antimetabolic, specifically a folic acid antagonist that inhibits dihydrofolate reductase, methotrexate is immunosuppressive with therapeutic activity in breast cancer, osteogenic carcinoma, acute leukemia and non-Hodgkin’s lymphoma, as well as noninfectious uveitis.
• Ocular side effects. Up to a quarter of patients undergoing high-dose intravenous methotrexate therapy can develop ocular surface toxicity within the first two to seven days after starting treatment, but this is usually self-limiting and can be treated with supportive topical therapy and lubrication. The ocular surface effects can be more severe with intravitreal methotrexate injection, used in patients treated for intraocular lymphoma.
The intrathecal route of administration and long-standing low-dose methotrexate have been rarely associated with toxic posterior optic neuropathy.9,22 The nerve damage can be reversible if the medication is discontinued early enough. Folate supplementation can help prevent this devastating complication.
Platinum analogs
Cisplatin and carboplatin, both of which are available under multiple brand names, are platinum cytotoxic drugs used to treat solid malignancies, such as head and neck, lung and testicular cancers.
• Ocular side effects. Cases of retinal damage in the form of cone dysfunction, as well as ischemic retinopathy, optic nerve edema and macular edema have been reported with these medications, but they’re not seen commonly.23,24 Cisplatin has been documented to produce neurotoxicity in the form of optic neuritis that can take occur with high-dose as well as cumulative-dose regimens and macular pigmentary changes, which is one of the few irreversible ophthalmic findings.22,25 Oncologists avoid intracarotid administration of cisplatin because of the potential for high ocular toxicity, including retrobulbar neuritis and central retinal artery occlusion.22
Bottom line
The wide array of novel immunotherapy and targeted therapy agents and chemotherapy drugs that have been introduced to treat a diverse range of malignancies has been revolutionary for the field of oncology. These medications can have distinct systemic effects, but we continue to add to our knowledge of their ophthalmic adverse effects as well. Ocular changes from some of these medications include serous retinopathy, macular edema, uveitis, optic nerve edema and surface disease.
Many of these findings can be managed while the patient continues with their potentially life-saving anticancer treatment, although in vision-threatening cases, the medication may need to be discontinued. Communication and partnership between the oncologist, ophthalmologist and other specialists is vital for optimal clinical outcomes. RS
REFERENCES
1. Kheir WJ, Sniegowski MC, El-Sawy T, Li A, Esmaeli B. Ophthalmic complications of targeted cancer therapy and recently recognized ophthalmic complications of traditional chemotherapy. Surv Ophthalmol. 2014;59:493-502.
2. Bindiganavile SH, Bhat N, Lee AG, Gombos DS, Al-Zubidi N. Targeted cancer therapy and its ophthalmic side effects: A review. J Immunother Precis Oncol. 2021;4:6-15.
3. Wladis EJ, Kambam ML. Ophthalmic complications of immune checkpoint inhibitors. Orbit. 2022;41:28-33.
4. Dalvin LA, Shields CL, Orloff M, Sato T, Shields JA. Checkpoint inhibitor immune therapy: Systemic indications and ophthalmic side effects. Retina. 2018;38:1063-1078.
5. Dow ER, Yung M, Tsui E. Immune checkpoint inhibitor-associated uveitis: Review of treatments and outcomes. Ocul Immunol Inflamm. 2021;29:203-211.
6. Crews J, Agarwal A, Jack L, Xu D, Do DV, Nguyen QD. Ipilimumab-associated retinopathy. Ophthalmic Surg Lasers Imaging Retina. 2015;46:658-660.
7. Mukhtar S, Jhanji V. Effects of systemic targeted immunosuppressive therapy on ocular surface. Curr Opin Ophthalmol. 2022;33:311-317.
8. Abdel-Rahman O, Oweira H, Petrausch U, et al. Immune-related ocular toxicities in solid tumor patients treated with immune checkpoint inhibitors: A systematic review. Expert Rev Anticancer Ther. 2017;17:387-394.
9. Liu CY, Francis JH, Abramson DH. Ocular side effects of systemically administered chemotherapy. 2023. UpToDate. Updated January 5, 2023. Available at: https://www.uptodate.com/contents/ocular-side-effects-of-systemically-administered-chemotherapy. Accessed March 2, 2023.
10. Dhillon AS, Hagan S, Rath O, Kolch W. MAP kinase signaling pathways in cancer. Oncogene. 2007;26:3279-3290.
11. Urner-Bloch U, Urner M, Jaberg-Bentele N, Frauchiger AL, Dummer R, Goldinger SM. MEK inhibitor-associated retinopathy (MEKAR) in metastatic melanoma: Long-term ophthalmic effects. Eur J Cancer. 2016;65:130-138.
12. Francis JH, Habib LA, Abramson DH, et al. Clinical and morphologic characteristics of MEK inhibitor–associated retinopathy: Differences from central serous chorioretinopathy. Ophthalmology. 2017;124:1788-1798.
13. Infante JR, Fecher LA, Falchook GS, et al. Safety, pharmacokinetic, pharmacodynamic, and efficacy data for the oral MEK inhibitor trametinib: A phase 1 dose-escalation trial. Lancet Oncol. 2012;13:773-781.
14. Mettler C, Monnet D, Kramkimel N, et al. Ocular safety profile of BRAF and MEK inhibitors: Data from the World Health Organization Pharmacovigilance Database. Ophthalmology. 2021;128:1748-1755.
15. Yin VT, Wiraszka TA, Tetzlaff M, Curry JL, Esmaeli B. Cutaneous eyelid neoplasms as a toxicity of vemurafenib therapy. Ophthalmic Plast Reconstr Surg. 2015;31:e112-115.
16. Eikenberry J, Harris A, Torabi R, et al. Ocular side effects of target therapy and immunotherapy in patients with cutaneous malignant melanoma. Eur J Ophthalmol. 2021;31:1391-1398.
17. Albertini GC, Corbelli E, Battaglia Parodi M, Bandello F. Choroidal neovascularization in multifocal choroiditis after dabrafenib and trametinib. Eur J Ophthalmol. 2017;27:e184-e186.
18. Choe CH, McArthur GA, Caro I, Kempen JH, Amaravadi RK. Ocular toxicity in BRAF mutant cutaneous melanoma patients treated with vemurafenib. Am J Ophthalmol. 2014;158831-837.e2.
19. Azarenko O, Smiyun G, Mah J, Wilson L, Jordan MA. Antiproliferative mechanism of action of the novel taxane cabazitaxel as compared with the parent compound docetaxel in MCF7 breast cancer cells. Mol Cancer Ther. 2014;13:2092-2103.
20. Fortes BH, Liou H, Dalvin LA. Ophthalmic adverse effects of taxanes: The Mayo Clinic experience. Eur J Ophthalmol. 2022;32:602-611.
21. Ye YT, Zhou ZY, Wen LS, Sun Y, Chu ZJ, Dou GR. The significance of the ocular adverse effect induced by systemic taxane application. Front Biosci (Landmark Ed). 2022;27:171.
22. Schmid KE, Kornek G v., Scheithauer W, Binder S. Update on ocular complications of systemic cancer chemotherapy. Surv Ophthalmol. 2006;51:19-40.
23. Dulz S, Asselborn NH, Dieckmann KP, et al. Retinal toxicity after cisplatin-based chemotherapy in patients with germ cell cancer. J Cancer Res Clin Oncol. 2017;143:1319-1325.
24. Wilding G, Caruso R, Lawrence TS, et al. Retinal toxicity after high-dose cisplatin therapy. J Clin Oncol. 1985;3:1683-1689.
25. Kupersmith MJ, Seiple WH, Holopigian K, Noble K, Hiesiger E, Warren F. maculopathy caused by intra-arterially administered cisplatin and intravenously administered carmustine. Am J Ophthalmol. 1992;113:435-438.



