 |
 |
The International Diabetes Federation estimated in 2017 that 8.5 percent of the world’s population is affected by diabetes mellitus.1 This translates to about 415 million people afflicted with DM currently and a projected 642 million people by 2040. All of them require screening examinations for the detection of diabetic retinopathy.
DR screening with timely treatment can prevent blindness. The American Academy of Ophthalmology recommends that patients with type 1 DM should first be screened within five years of onset and then yearly after 10 years duration.2 For type 2 DM patients, the AAO recommends DR screening at the time of diagnosis and then annually. Numerous studies support the association of earlier treatment with better outcomes.3,4 Yet, only a fraction of diabetes patients have DR screening. Several factors contribute to this low rate: lack of transportation; lack of insurance; noncompliance with recommendations and follow-up; and poor access to health care. Some of these are related to health-care disparities.5
This article looks at the latest research into artificial intelligence for DR and provides updates on the systems either available commercially or in the advanced stages of development.
Challenge of more DR screenings
Possible solutions to increase the proportion of patients getting DR screening include mobile screening units, remote telescreening using fundus photography6 and point-of-care screening using automated analysis of fundus photos with artificial intelligence networks.7 Mobile screening units are expensive and their access is limited to the geographical location of the mobile clinic. Tele-ophthalmology screening using fundus photography requires trained personnel to take photos and ophthalmologists to read the images. Since remote and multiple locations feed fundus photographs to the ophthalmologist, telemedicine has greater reach.
Use of retinal images in traditional telescreening results in a lag in relaying the results to the patient. In contrast, AI systems can “read” the fundus image within seconds and inform the patient quickly whether she needs further evaluation or if he or she can return for a screening visit in a year. Within minutes, these systems evaluate thousands of images to efficiently triage patients for referable diabetic retinopathy. AI systems that perform automated assessment of images would be more cost-effective, efficient and may also have improved accuracy compared to human grading.
In a systematic review of 18 studies, Francisco Pasquel, MD, and colleagues at Emory University evaluated the cost-effectiveness of various DR screening approaches.8 They concluded “the ideal screening technology must be portable, noninvasive, reliable and easy to use by relatively unskilled persons.” A Veterans Health Administration study also validated the cost-effectiveness of telescreening for DR.9 Although telemedicine was available to all patients in the primary-care clinics, only 40 to 55 percent had annual screenings, suggesting that there are still hurdles to overcome.10
Ease and efficiency of fundus images
Research has validated the use of fundus photos for DR screening. Initially 30-degree Early Treatment Diabetic Retinopathy (ETDRS) photos were shown to be comparable to retina specialists for the detection of DR.11 As early as 2011, automatic detection of DR was possible.12 More recently, 45-degree photos have been shown to be equivalent to seven-field ETDRS images.13 This simplifies the number of fundus photographs required for DR screening. Alternatives to 45-degree fundus photos are spectral-domain optical coherence tomography or OCT angiography images. However, these are significantly more expensive and studies have not yet validated these methods for detection of DR.
 |
Several AI systems designed to screen for DR utilize fundus photos. When evaluating these AI systems, sensitivity is indicative of safety and specificity of effectiveness. That is, a high sensitivity for detection of sight-threatening retinopathy is needed for the system to be safe. A high specificity is also needed so that the system can distinguish DR from other conditions and thus be an effective screening tool. Several AI systems are capable of detecting DR.
IDx-DR paves the way
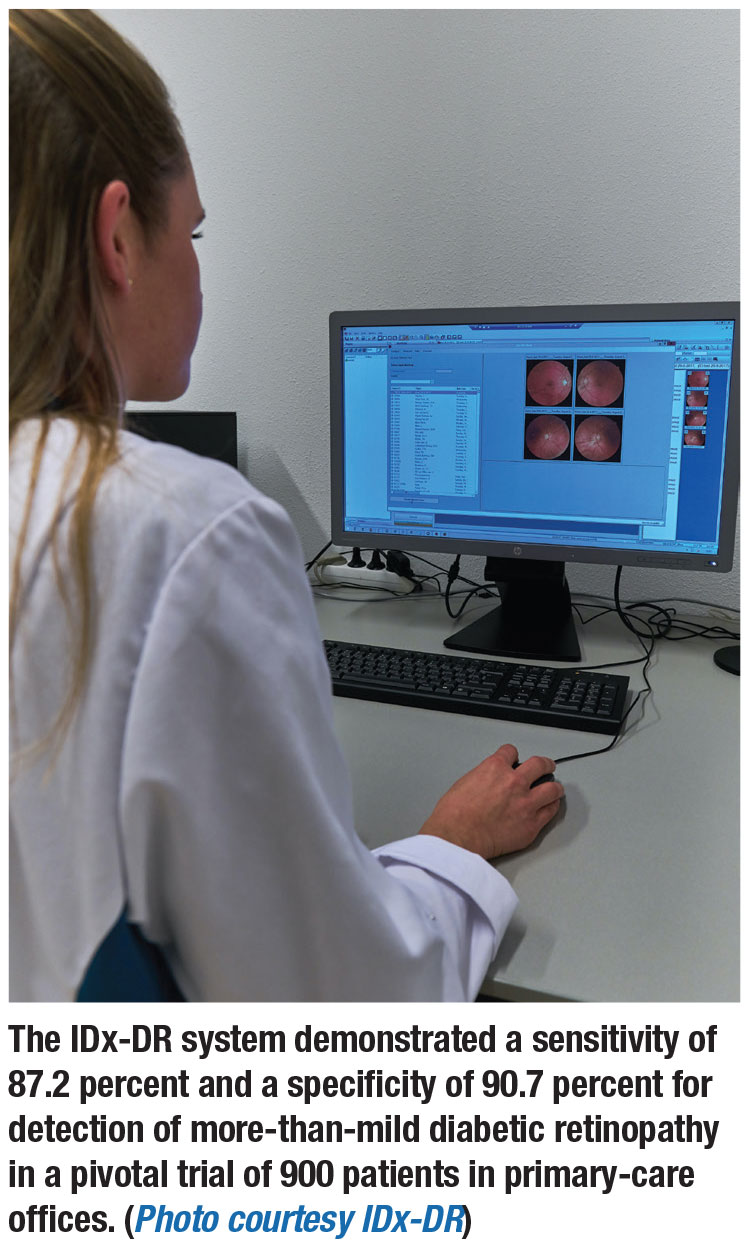
The IDx-DR system is the first commercially available AI diabetic screening system.14 Earlier versions of IDx-DR were evaluated as part of the Iowa Detection Program (IDP). With modifications to the IDP algorithms, improved sensitivity with maintained specificity resulted. The IDx-DR uses two 45-degree fundus images, which trained staff captures with a Topcon non-mydriatic fundus camera.
The IDx-DR algorithm determines the presence of more-than-mild DR (mtmDR), defined as ETDRS level 35 or higher and/or diabetic macular edema in at least one eye. Presence of mtmDR triggers a recommendation for ophthalmic evaluation.
A pivotal trial of 900 patients in primary-care offices found a sensitivity for
detection of mtmDR of 87.2 percent and a specificity of 90.7 percent.15 Study patients underwent IDx-DR imaging and four-widefield stereoscopic fundus photographs (equivalent to the area of the retina covered by the modified seven-field stereo film ETDRS protocol) and macular OCT. The study compared the detection rate of mtmDR with the IDx-DR system to the Wisconsin Fundus Photograph Reading Center (FRCP) certified retinal photographers’ readings of the reference standards (photos and OCT).
The Food and Drug Administration approved this first fully autonomous AI diagnostic system in 2018.16 IDX-DR systems have since been deployed to several locations and studies are ongoing to further evaluate its effectiveness. By collaborating with locations using the IDx-DR, investigators currently seek to find out whether patients who were advised to see an eye-care provider are doing so and being treated for DR.
Google Deep Mind
Google Deep Mind is a convolutional neural network-based DR-detection algorithm first published in 2016.17 Deep Mind showed a high sensitivity and specificity for detection of DR. This DR deep-learning (DL) algorithm was used on the Messidor-2 dataset as well as a retrospective dataset of 9,963 images taken as part of routine DR screening in the United States and India. The algorithm performed well compared to human graders (seven for the Messidor-2 and eight for the other set), using a majority decision as a reference standard for referable retinopathy.
 |
When evaluated on the Messidor-2 images, the DL algorithm, when tuned for high sensitivity, resulted in 96.1 percent sensitivity and 93.9 percent specificity. When tuned for high specificity, the sensitivity was 87 percent and specificity 98.5 percent. When used on the retrospective data set and tuned for high sensitivity, sensitivity was 97.5 percent and specificity 93.4 percent. When tuned for high specificity, sensitivity was 90.3 percent and specificity 98.1 percent. These results are quite good and suggest both safety in detecting referable DR and efficacy in that false positives were low (1-specificity).
A nationwide DR screening program in Thailand validated the Google DL algorithm in a community setting.18 A total of 25,326 gradable retinal images from 7,517 patients with diabetes were analyzed for different DR severity levels and macular edema. The algorithm was improved from detection of referable and non-referable DR into detection of the five severity levels of DR. This study performed grading adjudication among an international group of retinal specialists with a majority consensus serving as the reference standard.
Compared with human graders, the algorithm had higher sensitivity across all severity levels of DR and DME (p< 0.001). For detecting different severity levels for referrals (moderate nonproliferative DR, severe NPDR, proliferative DR and DME), the algorithm also had significantly higher sensitivity (0.97 vs. 0.74, p<0.001) and slightly lower specificity (0.96 vs. 0.98, p<0.001). False negatives were reduced by 23 percent at the cost of a 2 percent increase in false positives.
EyeArt System
Another DL-based system is the EyeArt system (Eyenuk). This system of multiple deep-neural networks for specific classification tasks on images was trained on 375,000 images and validated on 250,000. EyeArt uses cloud-based software and has an application that can interface with existing imaging and telescreening software.
The system analyzes 45-degree fundus photos to determine the presence of referable DR within 60 seconds. It uses the International Classification of Diabetic Retinopathy (ICDR) definition of greater than or equal to moderate NPDR and or clinically significant DME to define referable DR. It also generates a recommendation report regarding patient disposition, referral or return for future screening.
The EyeArt system was studied on smartphone app-based fundus images. The study analyzed retinal images of 296 patients taken with a Remidio Fundus on Phone device. Even though the EyeArt algorithms were not trained on smartphone-based fundus photography, EyeArt achieved a sensitivity of 95.8 percent for any DR, 99.3 percent for referable DR and 99.1 percent for sight-threatening DR, with specificities of 80.2, 68.8 and 80.4 percent, respectively.19
A point-of-care prospective study at 15 sites (primary care, endocrinology, ophthalmology and retina offices) recently evaluated EyeArt to screen for referable DR. Adult patients were included if they had a diagnosis of DM and excluded if they had known other retinal vascular disease, documented DME, prior retinal laser or prior eye surgery other than cataract extraction. In this study, non-mydriatic (or mydriatic images, mydriasis allowed if needed), 45-degree, two-field fundus photos were uploaded to the cloud for determination of referable DR. The system’s determination of referable DR was compared to four 45-degree, widefield stereoscopic mydriatic photos read by the Wisconsin FPRC.
Of the 1,830 images, 1,674 were gradable by both the EyeArt System and the reading center. Of 1,364 eyes negative for referable DR, EyeArt correctly identified 1,180 with 184 false positives, which were composed mostly of mild NPDR. Of 310 eyes positive for referable DR, EyeArt correctly identified 296 of them. False negatives were all composed of level 35 moderate NPDR. Sensitivity was 96 percent, specificity 86 percent and gradability 87.5 percent, increasing to 97.4 percent with dilation as needed.
 |
SERI-NUS System
The Singapore Eye Research Institute (SERI) has developed and validated the SERI-NUS DL system on more than 500,000 fundus images.20 21 This system has shown a sensitivity of 90.5 percent and specificity of 91.3 percent for detection of referable DR compared to 91.6 percent sensitivity and 99.3 percent specificity by professional graders.22 For detection of sight-threatening DR, sensitivity was 100 percent and specificity 91.1 percent compared to 88.6 percent sensitivity and 99.6 percent specificity of human graders.
Unlike other systems, SERI-NUS also has algorithms for detecting glaucoma suspects (cup-to-disc ratio >0.8 or presence of optic disc hemorrhage or notching), or intermediate or higher AMD. Sensitivity and specificity were 96.4 and 87.2 percent, respectively, for the glaucoma algorithm, and 93.2 and 88.7 percent, respectively, for the AMD algorithm. Area under the curve was high for both (0.942 for glaucoma and 0.931 for AMD)—important as these patients after a certain age need yearly check-ups.
Bottom line
The current DL algorithms have demonstrated both high sensitivity (safety) and high specificity (effectiveness) and thus show that they can enable point-of-care DR screening. This will be invaluable in the future for triage and identification of DR patients.
Refinements in these algorithms and perhaps additional programming to screen for other conditions will make the programs even more powerful in screening for DR and associated ocular conditions.
AI will transform the management of patients. It will lead to more efficient use of our health-care workers and resources while also achieving increased detection of DR in patients. Properly deployed, it can reduce disparities in health care in terms of disease detection. Ultimately the true test of AI’s effectiveness will be whether or not more patients with referable DR are indeed seen and cared for by ophthalmologists. Compared to telescreening, AI has been shown to be cost-effective. Perhaps the saved health-care dollars from its use can then be applied toward the cost of ophthalmic procedures to treat DR and prevent blindness. RS
REFERENCES
1. Ogurtsova K, da Rocha Fernandes JD, Huang Y, et al. IDF Diabetes Atlas: global estimates for the prevalence of diabetes for 2015 and 2040. Diabetes Res Clin Pract. 2017;128:40–50.
2. Flaxel CJ, Adelman RA, Bailey ST, et al. Diabetic retinopathy preferred practice pattern. Ophthalmology. 2019;127:66-145.
3. Early Treatment Diabetic Retinopathy Study Research Group. Fundus photographic risk factors for progression of diabetic retinopathy. ETDRS report number 12. Ophthalmology. 1991;98(suppl):823S-833S.
4. The Diabetic Retinopathy Study Research Group. Four risk factors for severe visual loss in diabetic retinopathy: The third report from the Diabetic Retinopathy Study. Arch Ophthalmol. 1979;97:654-655.
5. Peek ME, Cargill A, Huang ES. Diabetes health disparities: a systematic review of health care interventions. Med Care Res Rev. 2007;64(suppl):101S-156S.
6. Sanchez CR, Silva PS, Cavallerano JD, Aiello LP, Aiello LM. Ocular telemedicine for diabetic retinopathy and the Joslin Vision Network. Semin Ophthalmol. 2010;25:218-224.
7. Silva PS, Cavallerano JD, Aiello LM, Aiello LP. Telemedicine and diabetic retinopathy: moving beyond retinal screening. Arch Ophthalmol. 2011;129:236-242.
8. Pasquel FJ, Hendrick AM, Ryan M, Cason E, Ali MK, Narayan KM. Cost-effectiveness of different diabetic retinopathy screening modalities. J Diabetes Sci Technol. 2015;10:301-307.
9. Kirkizlar E, Serban N, Sisson JA, Swann JL, Barnes CS, Williams MD. Evaluation of telemedicine for screening of diabetic retinopathy in the Veterans Health Administration. Ophthalmology. 2013;120:2604-2610.
10. Mansberger SL, Sheppler C, Barker G, et al. Long-term comparative effectiveness of telemedicine in providing diabetic retinopathy screening examinations: A randomized clinical trial. JAMA Ophthalmol. 2015;133:518–525.
11. Cavallerano JD, Aiello LP, Cavallerano AA, et al., for the Joslin Vision Network Clinical Team. Nonmydriatic digital imaging alternative for annual retinal examination in persons with previously documented no or mild diabetic retinopathy. Am J Ophthalmol. 2005;140:667-673.
12. Agurto C, Barriga ES, Murray V, et al. Automatic detection of diabetic retinopathy and age-related macular degeneration in digital fundus images. Invest Ophthalmol Vis Sci. 2011;52:5862-5871.
13. Gangaputra S, Almukhtar T, Glassman AR, et al, for the Diabetic Retinopathy Clinical Research Network. Comparison of film and digital fundus photographs in eyes of individuals with diabetes mellitus. Invest Ophthalmol Vis Sci. 2011;52:6168-6173.
14. Abràmoff MD, Lou Y, Erginay A, et al. Improved automated detection of diabetic retinopathy on a publicly available dataset through integration of deep learning. Invest Ophthalmol Vis Sci. 2016;57:5200–5206.
15. Abràmoff MD, Lavin PT, Birch M, Shah N, Folk JC. Pivotal trial of an autonomous AI-based diagnostic system for detection of diabetic retinopathy in primary care offices. NPJ Digit Med. 2018;1:39.
16. FDA permits marketing of artificial intelligence-based device to detect certain diabetes-related eye problems [news release]. Bethesda, MD; U.S. Food and Drug Administration; April 11, 2018.
17. Gulshan V, Peng L, Coram M, Stumpe MC, Wu D, Narayanaswamy A, et al. Development and validation of a deep learning algorithm for detection of diabetic retinopathy in retinal fundus photographs. JAMA 2016;316:2402–10.
18. Raumviboonsuk P, Krause J, Chotcomwongse P, et al. Deep learning versus human graders for classifying diabetic retinopathy severity in a nationwide screening program. NPJ Digit Med. 2019;2:25.
19. Rajalakshmi R, Subashini R, Anjana RM, Mohan V. Automated diabetic retinopathy detection in smartphone-based fundus photography using artificial intelligence. Eye. 2018;32:1138-1144.
20. Ting DSW, Peng L, Varadarajan AV, et al. Deep learning in ophthalmology: The technical and clinical considerations. Prog Retin Eye Res. 2019;72:100759.
21. Cheung CY, Tang F, Ting DSW, Tan GSW, Wong TY. Artificial intelligence in diabetic eye disease screening. Asia Pac J Ophthalmol (Phila). 2019 Apr 24. doi:10.22608/APO.201976. [Epub ahead of print]
22. Ting DSW, Cheung CY-L, Lim G, et al. Development and validation of a deep learning system for diabetic retinopathy and related eye diseases using retinal images from multiethnic populations with diabetes. JAMA. 2017;318:2211–2223.
23. Ribeiro ML, Nunes SG, Cunha-Vaz JG. Microaneurysm turnover at the macula predicts risk of development of clinically significant macular edema in persons with mild nonproliferative diabetic retinopathy. Diabetes Care. 2012;36:1254–1259.
24. Tufail A, Rudisill C, Egan C, et al. Automated diabetic retinopathy image assessment software: diagnostic accuracy and cost-effectiveness compared with human graders. Ophthalmology. 2017;124:343-351.
25. Alam M, Zhang Y, Lim JI, Chan, RVP, Yang M, Yao X. Quantitative optical coherence tomography angiography features for objective classification and staging of diabetic retinopathy. Retina. 2018 Oct 31. doi:10.1097/IAE.0000000000002373 [Epub ahead of print].
26. Ashraf M, Nesper PL, Jampol LM, Yu F, Fawzi AA. Statistical model of optical coherence tomography angiography parameters that correlate with severity of diabetic retinopathy. Invest Ophthalmol Vis Sci. 2018;59:4292–4298.



