Take-home points
|
 |
|
Bios Dr. Garg is an ophthalmology resident at the Wilmer Eye Institute of Johns Hopkins University in Baltimore. Dr. Campbell is an associate professor of ophthalmology at the Casey Eye Institute of Oregon Health and Science University, Portland. Dr. Kashani is an associate professor and the Boone Pickens Professor of Ophthalmology at the Wilmer Eye Institute. DISCLOSURES: Dr. Garg and Dr. Campbell have no relevant disclosures. Dr. Kashani disclosed a relationship with Carl Zeiss Meditec. |
Optical coherence tomography, introduced in 1991,1 has had a transformational impact on the diagnosis and management of macular pathology such as choroidal neovascularization and macular edema. In short order the application of OCT to diseases of the vitreoretinal interface also rapidly evolved.2,3
With these applications, the importance of peripheral retinal imaging also emerged when investigators began to use montages of spectral-domain OCT images4 or larger scan patterns5 to explore vitreoretinal interface findings outside the macula. These studies revealed that OCT of the retinal periphery was a significantly underutilized application that could provide important diagnostic information. However, the practical limitations in acquiring and montaging OCT images from outside the macula largely precluded the routine use of OCT for peripheral retinal pathology.
Over the past decade, the adoption of high-speed and ultrahigh-speed swept-source OCT technology has made it easier to image outside the macula—known as “widefield” imaging (WFI).6 Consensus terminology for WFI has been developed both in the context of fundus imaging as well as OCT7 and the pathophysiologic importance of imaging the periphery is widely acknowledged.8 The increased scanning speeds (up to 100,000 A scans/s) of commercially available SS-OCT platforms enable acquisition of fields of view up to approximately 12-x-12 mm in a single-scan pattern in few seconds (Table).
The age of OCT angiography
More recently, OCT angiography has enabled high-resolution, noninvasive visualization of the retinal and choroidal microvasculature with distinct advantages over fluorescein angiography,9 including the absence of intravenous dye injection. OCTA provides capillary level resolution of the retinal vasculature and enables visualization of the peripapillary radial capillaries as well as the deep-retinal capillaries that conventional dye-based angiography doesn’t visualize.
In addition, the speed and ease of use of commercially available WF-OCT and WF-OCTA systems allow for comfortable and rapid imaging of patients at each visit to closely monitor disease progression and therapy response.10,11 In this article, we will review the currently available commercial WF-OCT and WF-OCTA systems as well as experimental concept instruments to illustrate clinical applications in adults and children. The accompanying table summarizes characteristics of several commercially available widefield OCT and OCTA systems.
 |
While there are many clinical applications for WF-OCT and WF-OCTA, at least three have immediate clinical relevance and impact: diabetic retinopathy; retinal vein occlusion; and pediatric uses. Recent peer-reviewed clinical studies demonstrate and support these applications, but much room exists for improvement.
 |
|
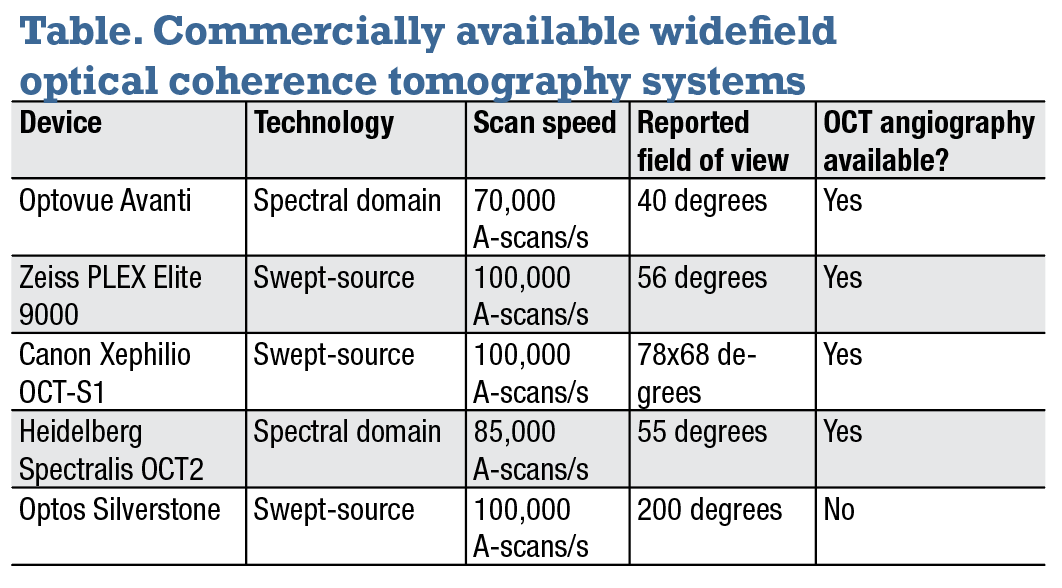
Figure 1. Widefield optical coherence tomography angiography of two patients with diabetic retinopathy illustrating regions of neovascularization with corresponding B-scans. A) En face 12-x-12mm field of view depth encoded pseudocolored OCTA of the right eye of a person with clinically apparent proliferative DR. B) Horizontal B-scan (corresponding to the white line in panel A), demonstrates retinal neovascularization in the vitreous. C) En face WF-OCTA of a patient with severe nonproliferative DR and (D) corresponding B-scan of the same patient with presumed severe NPDR demonstrate flat neovascularization above the internal limiting membrane that wasn't detected clinically. OCTA is particularly effective at identifying this flat neovascularization that can be very difficult to detect clinically. (Color coding for panels A and B: Red=superficial retinal layer; green=deep retinal layer; yellow=overlay of red/green. (Images obtained on Zeiss PlexElite 9000, Carl Zeiss Meditec) |
Diabetic retinopathy
Widefield fundus imaging has already demonstrated that the perfusion status of the retinal periphery is particularly important for patients with diabetic retinopathy because of an increased risk of DR progression when predominantly peripheral lesions are present.12,13 Multiple studies have also shown the ability of WF-OCTA to identify regions of nonperfusion and neovascularization in patients with DR.14,15
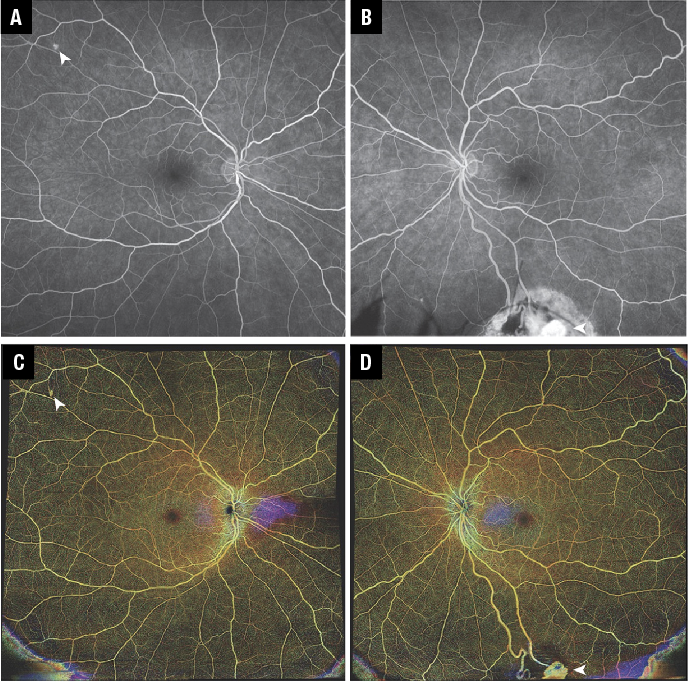
In some studies, the ability of WF-OCTA to detect regions of impaired perfusion is debatably superior to that of FA.15,16 Figure 1 demonstrates the capillary level resolution of WF-OCT images that highlight areas of impaired perfusion. Notably, these areas aren’t clinically evident on dilated fundus examinations. Or in some cases FA and the benefit of imaging is significant in early identification of impaired perfusion. Figure 2 is a montage of several WFIs demonstrating even larger field-of-view capabilities likely to be commercially available in the near future.A separate study used FA and WF-OCTA to detect regions of nonperfusion in nine patients with DR. Notably, the study found that all regions of nonperfusion detectable on FA were also detected on WF-OCTA, but that WF-OCTA could identify additional regions not detectable on FA.15 A 2020 study of 20 eyes also demonstrated this finding,17 observing that areas of nonperfusion could be more easily identified using WF-OCTA in eyes that had been treated with panretinal photocoagulation because of the appearance of PRP scars on FA.
A 2022 study in Japan used WF-OCTA to quantify the progression of neovascularization using swept-source widefield OCTA images.18 The authors demonstrated the ability of widefield OCTA to not only identify, but also track the progression of neovascularization lesions. Figures 1C and D (page 31) illustrate a case where numerous areas of neovascularization are evident on WF-OCTA but weren’t recognized on clinical examination.
 |
|
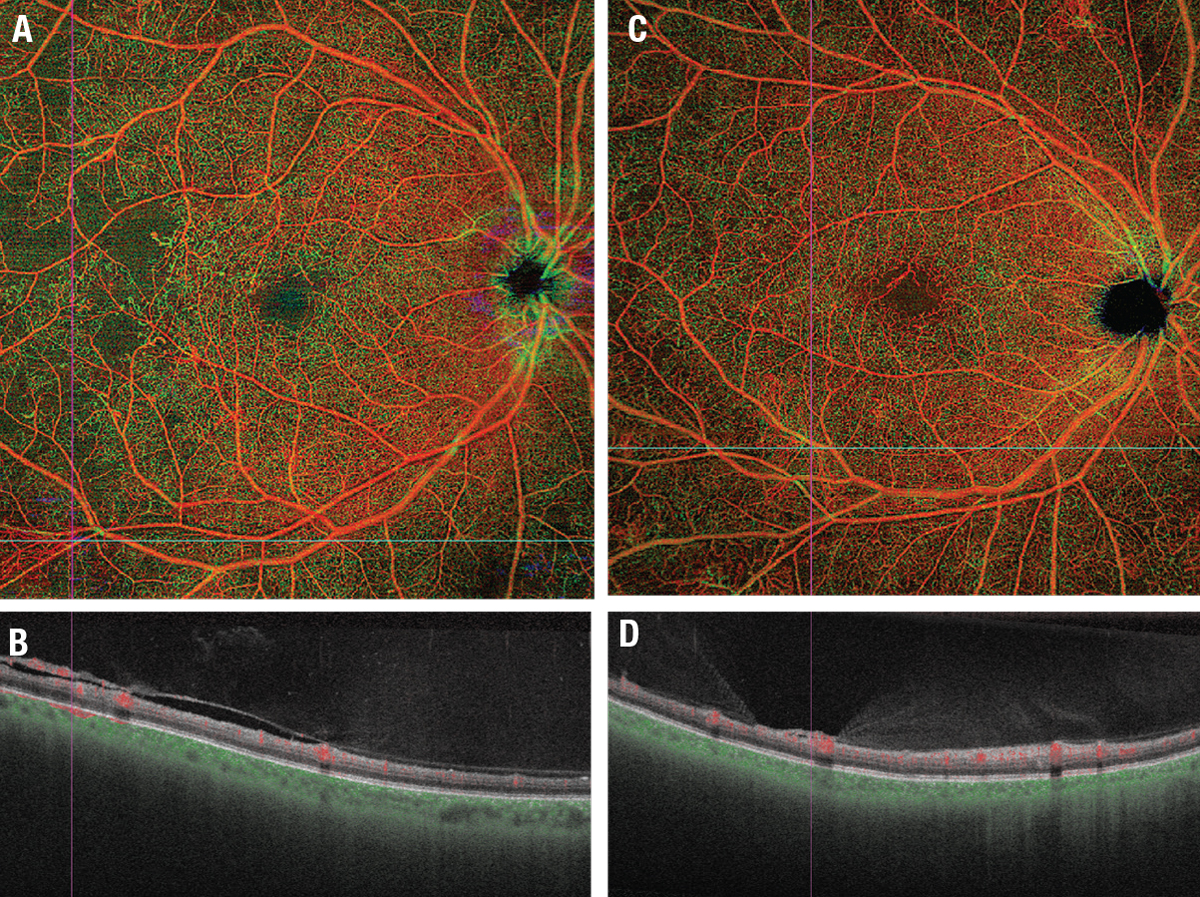
Figure 2. Wide-field montage of several images demonstrating visualization of peripheral retina. This figure provides an expanded field-of-view of the same patient imaged in Figure 1A and B and illustrates additional impaired temporal perfusion in the mid-periphery not seen in Figure 1. This montage feature is not yet Food and Drug Administration-approved. (Obtained on Zeiss PlexElite 9000, Carl Zeiss Meditec) |
Retinal vein occlusion
Recent work has demonstrated the utility of WF-OCTA in assessment of nonperfusion in patients with RVO. A study from France last year comparing ultra-WF FA and WF-OCTA in patients with RVO calculated that WF-OCTA had a 100 percent sensitivity and 65 percent specificity for detection of nonperfusion, indicating that it may serve as an effective screening tool for patients with RVO.19
Another study retrospectively evaluated 39 eyes with ischemic RVO to detect neovascularization elsewhere as well as areas of nonperfusion.20 WF-OCTA detected 100 percent of areas of nonperfusion and retinal neovascularization that FA had detected. In one case, neovascularization elsewhere found on OCTA wasn’t detectable using FA. A separate study compared the area of nonperfusion between WF-OCTA and FA in 27 eyes with BRVO and found a strong and statistically significant correlation between the two modalities.21
Pediatric patients
In pediatric retina, OCT imaging is typically limited to cooperative children who can position themselves into imaging systems designed for adults or patients in the operating room under anesthesia.22,23 Under anesthesia, camera options are limited, particularly for OCT, which often isn’t available. Furthermore, commercially available options for imaging under anesthesia are typically slower than those used in routine clinical practice.
Many pediatric retinal diseases have complicated vitreoretinal interface abnormalities and peripheral vascular abnormalities likely to benefit from OCT imaging. However, they’re difficult to visualize using available portable OCT systems that have a limited field of view.24 This limitation has delayed our understanding of both normal pediatric macular development and pathologic changes with disease.
Finally, diseases such as retinopathy of prematurity require repeated clinical examination at the bedside, which often occur without the benefits of imaging, especially OCT.25 Some of these challenges can be overcome with improved optical designs and faster lasers that can facilitate more efficiency, higher resolution and greater field-of-view compared with currently available OCT systems.26 We present a series of pediatric patients (Figure 3) imaged using a prototype 400 kHZ swept-source OCT with a field of view of up to 240 degrees to demonstrate the feasibility and potential clinical benefit of incorporating WF-OCT into the pediatric retinal practice in the future.27
 |
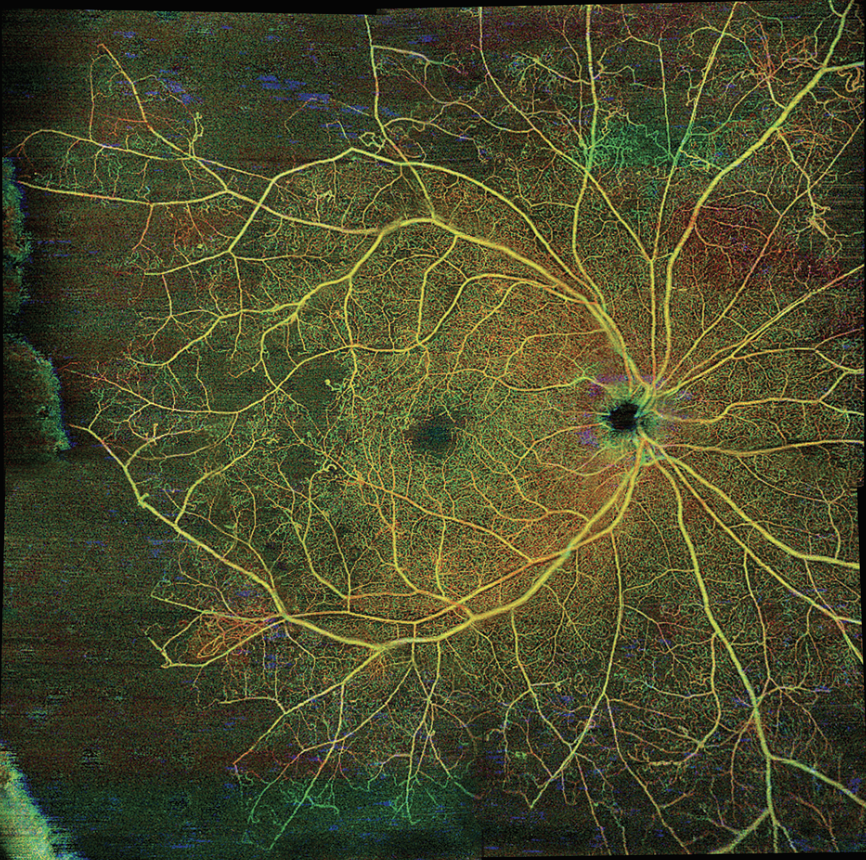
| Figure 3. Examples of peripheral pathology visualized with ultra-widefield en face optical coherence tomography. All scans were acquired in 1.5 seconds using a prototype contact UWF handheld OCT system. A, B) Pediatric patients imaged with UWF OCT in the operating room. Patient A had Von Hippel Lindau syndrome and multiple hemangioblastomas, including one near the ora serrata. Patient B was diagnosed with retinoblastoma, which could be more effectively captured using UWF OCT than commercially available systems with lower fields of view. C, D) Retinopathy of prematurity seen on images obtained at the bedside in the neonatal intensive care unit. (Courtesy Yifan Jian, PhD) |
Future applications and directions
As WF-OCT and OCTA devices continue to improve and are adopted into clinical practice, new research and clinical applications will undoubtedly emerge. Figure 4 provides a striking example of retinal hemangioblastomas imaged with a WF-OCTA montage, which isn’t Food and Drug Administration-approved, compared with FA. In mouse models, WF-OCT has been combined with adaptive optics scanning laser ophthalmoscopy to localize individual cells and structures at a three-dimensional level, which may assist with precisely tracking cellular structure in vivo to better understand retinal pathologies.28
 |
|
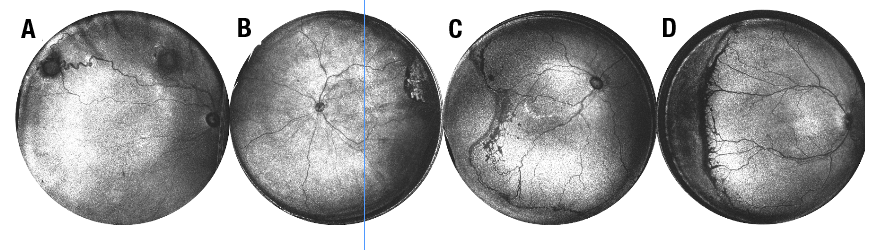
Figure 4. A 34-year-old woman with a history of pheochromocytoma, pancreatic tumors, central-nervous-system and retinal hemangioblastomas secondary to Von Hippel-Lindau syndrome underwent optical coherence tomography angiography imaging. She was diagnosed at age 24 based on ocular manifestations and confirmed with genetic testing (both parents tested negative). Visual acuity was 20/20 bilaterally. A) Fluorescein angiography of the right eye demonstrates small untreated capillary hemangioblastoma (arrowhead) temporally superior to the macula. B) FA of the left eye showed a large inferior hemangioblastoma (treated with cryotherapy nine years prior) with persistent flow. C, D) 12-x-12-mm OCTA montages reveal lesions consistent with hemangioblastomas identified in figures A and B. (Images obtained on Zeiss PlexElite 9000, Carl Zeiss Meditec) |
Importantly, as widefield imaging has become ubiquitous, an effort is being made to standardize nomenclature when discussing widefield imaging devices. The International Widefield Imaging Study Group, which was composed of several leading retinal specialists around the world, proposed a consistent nomenclature for widefield OCTA imaging, recommending that the term “widefield” be limited to images including all four quadrants as well as the posterior edge of the vortex vein ampulla.7 These efforts to standardize imaging nomenclature will be critical as the body of literature utilizing widefield imaging continues to grow.
Bottom Line
Since its introduction in 1991, OCT has become a transformational tool for clinical diagnosis and management of retinal diseases primarily involving the macula. Advances in OCT technology have enabled WF-OCT and, more recently, WF-OCTA that allow extramacular imaging of the posterior pole and even mid-to-far periphery. These technologies have enabled accurate and reliable, noninvasive, commercially available imaging platforms.
Improvements such as montaging features are expected in the near future and will continue to expand the applications and potential of WFI systems. Even now, a steady stream of studies has demonstrated WF-OCTA can reliably identify clinically relevant lesions in DR, RVO and other retinal vascular disorders with similar efficacy to dye-based angiography and with less inconvenience to staff and patients. Logistical and financial barriers to wide adoption of widefield imaging platforms still exist, but their benefit and potential are compelling enough that their adoption seems inevitable. RS
References
1. Huang D, Swanson EA, Lin CP, et al. Optical coherence tomography. Science. 1991;254:1178-1181.
2. Witkin AJ, Ko TH, Fujimoto JG, et al. Redefining lamellar holes and the vitreomacular interface: An ultrahigh-resolution optical coherence tomography study. Ophthalmology. 2006;113:388-397.
3. Duker JS, Kaiser PK, Binder S, et al. The international vitreomacular traction study group classification of vitreomacular adhesion, traction, and macular hole. Ophthalmology. 2013;120:2611-2619.
4. Mori K, Kanno J, Gehlbach PL, Yoneya S. Montage images of spectral-domain optical coherence tomography in eyes with idiopathic macular holes. Ophthalmology. 2012;119:2600-2608.
5. McNabb RP, Grewal DS, Mehta R, et al. Wide field of view swept-source optical coherence tomography for peripheral retinal disease. Br J Ophthalmol. 2016;100:1377-1382.
6. Miller AR, Roisman L, Zhang Q, et al. Comparison between spectral-domain and swept-source optical coherence tomography angiographic imaging of choroidal neovascularization. Investig Ophthalmol Vis Sci. 2017;58:1499-1505.
7. Choudhry N, Duker JS, Freund KB, et al. Classification and guidelines for widefield imaging: Recommendations from the International Widefield Imaging Study Group. Ophthalmol Retin. 2019;3:843-849.
8. Quinn N, Csincsik L, Flynn E, et al. The clinical relevance of visualising the peripheral retina. Prog Retin Eye Res. 2019;68:83-109. doi:10.1016/j.preteyeres.2018.10.001
9. Schwartz DM, Fingler J, Kim DY, et al. Phase-variance optical coherence tomography: A technique for noninvasive angiography. Ophthalmology. 2014;121:180-187.
10. Borrelli E, Uji A, Toto L, Viggiano P, Evangelista F, Mastropasqua R. In vivo mapping of the choriocapillaris in healthy eyes: A widefield swept-source OCT angiography study. Ophthalmol Retin. 2019;3:979-984.
11. Kolb JP, Klein T, Kufner CL, Wieser W, Neubauer AS, Huber R. Ultra-widefield retinal MHz-OCT imaging with up to 100 degrees viewing angle. Biomed Opt Express. 2015;6:1534.
12. Silva PS, Dela Cruz AJ, Ledesma MG, et al. Diabetic retinopathy severity and peripheral lesions are associated with nonperfusion on ultrawide field angiography. Ophthalmology. 2015;122:2465-2472.
13. Silva PS, Cavallerano JD, Haddad NMN, et al. Peripheral lesions identified on ultrawide field imaging predict increased risk of diabetic retinopathy progression over 4 years. Ophthalmology. 2015;122:949-956.
14. Sawada O, Ichiyama Y, Obata S, et al. Comparison between wide-angle OCT angiography and ultra-wide field fluorescein angiography for detecting non-perfusion areas and retinal neovascularization in eyes with diabetic retinopathy. Graefe’s Arch Clin Exp Ophthalmol. 2018;256(7):1275-1280.
15. Couturier A, Rey PA, Erginay A, et al. Widefield OCT-angiography and fluorescein angiography assessments of nonperfusion in diabetic retinopathy and edema treated with anti–vascular endothelial growth factor. Ophthalmology. 2019;126:1685-1694.
16. Pellegrini M, Cozzi M, Staurenghi G, Corvi F. Comparison of wide field optical coherence tomography angiography with extended field imaging and fluorescein angiography in retinal vascular disorders. PLoS One. 2019;14:1-9.
17. Russell JF, Al-khersan H, Shi Y, et al. Retinal nonperfusion in proliferative diabetic retinopathy before and after panretinal photocoagulation assessed by widefield OCT angiography. Am J Ophthalmol. 2020;213:177-185. doi:10.1016/j.ajo.2020.01.024
18. Shiraki A, Sakimoto S, Eguchi M, et al. Analysis of progressive neovascularization in diabetic retinopathy using widefield OCT angiography. Ophthalmol Retin. 2022;6:153-160.
19. Glacet-Bernard A, Miere A, Houmane B, Tilleul J, Souied E. Nonperfusion assessment in retinal vein occlusion: comparison between ultra-widefield fluorescein angiography and widefield optical coherence tomography angiography. Retina. 2021;41:1202-1209.
20. Huemer J, Khalid H, Wagner SK, et al. Phenotyping of retinal neovascularization in ischemic retinal vein occlusion using wide field OCT angiography. Eye. 2021;35:2812-2819.
21. Shiraki A, Sakimoto S, Tsuboi K, et al. Evaluation of retinal nonperfusion in branch retinal vein occlusion using wide-field optical coherence tomography angiography. Acta Ophthalmol. 2019;97:e913-e918.
22. Kunkler AL, Patel NA, Russell JF, et al. Intraoperative OCT angiography in children with incontinentia pigmenti. Ophthalmol Retin. 2022;6:330-332.
23. Basilius J, Young MP, Michaelis TC, Hobbs R, Jenkins G, Hartnett ME. Structural abnormalities of the inner macula in incontinentia pigmenti. JAMA Ophthalmol. 2015;133:1067-1072. doi:10.1001/jamaophthalmol.2015.1700
24. He Y, Chen X, Tsui I, Vajzovic L, Sadda SR. Insights into the developing fovea revealed by imaging. Prog Retin Eye Res. Published online May 17, 2022. doi:10.1016/j.preteyeres.2022.101067
25. Scruggs BA, Ni S, Nguyen T-TP, et al. Peripheral OCT assisted by scleral depression in retinopathy of prematurity. Ophthalmol Sci. 2022;2:100094.
26. Ni S, Nguyen T-TP, Ng R, et al. Ultra-widefield handheld swept-source OCT for peripheral retinal imaging. Proc. SPIE 11941, Ophthalmic Tech XXXII, 1194109 (4 March 2022); doi:10.1117/12.2608284
27. Nguyen TP, Ni S, Liang G, et al. Widefield optical coherence tomography in pediatric retina: A case series of intraoperative applications using a prototype handheld device. Front Med (Lausanne). 2022;9:860371.
28. Zawadzki RJ, Zhang P, Zam A, et al. Adaptive-optics SLO imaging combined with widefield OCT and SLO enables precise 3D localization of fluorescent cells in the mouse retina. Biomed Opt Express. 2015;6:2191.



