| In Brief • Second Sight Medical Products Inc., developer of the Argus II retinal prosthesis system, announced the first successful implantation and activation of a wireless visual cortical stimulator in a human, providing the initial human proof of concept for the ongoing development of the Orion Visual Cortical Prosthesis. Surgeons at UCLA implanted the wireless multichannel neurostimulation system on the visual cortex of the 30-year-old recipient, who then could perceive and localize individual phosphenes with no significant side effects. • Applied Genetic Technologies Corporation (AGTC) filed an investigational new drug application with the U.S. Food and Drug Administration to conduct a Phase I/II clinical trial of its second gene therapy candidate for the treatment of achromatopsia—this one for mutations in the CNGA3 gene. AGTC already has a Phase I/II clinical trial under way of its gene therapy candidate for achromatopsia caused by mutations in the CNGB3 gene. The company plans to initiate the trial of the CNGA3 therapy in the coming months. • Genentech has received FDA approval of the Lucentis (ranibizumab) 0.5 mg prefilled syringe for treatment of age-related macular degeneration and macular edema after retinal vein occlusion. |
“There is no doubt in my mind that we have just witnessed a vision of eye surgery in the future,” Prof. MacLaren said after completing the first macular peel. “Current technology with laser scanners and microscopes allows us to monitor retinal diseases at the microscopic level, but the things we see are beyond the physiological limit of what the human hand can operate on. With a robotic system, we open up a whole new chapter of eye operations that currently cannot be performed.”
The robot accesses the surgical area in the eye through a single port less than 1 mm in diameter. It uses seven independent computer-controlled motors to carry out hand movements as precise as 0.001 mm in scale. The surgeon uses a joystick and touchscreen at a control console to manipulate the robotic probe inside the eye, viewing the surgical area through the operating microscope.
The robot can be a breakthrough for delivering gene therapy to the back of the eye, Prof. MacLaren said. “This will help to develop novel surgical treatments for blindness, such as gene therapy and stem cells, which need to be inserted under the retina with a high degree of precision,” he said.
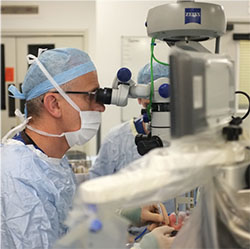 |
| Robert E. MacLaren, MBChB, conducts the first macular peel using the Robotic Retinal Dissection Device at University of Oxford’s John Radcliffe Hospital. Credit: University of Oxford |
Vector Gene Transfer for NVAMD Sustains Expression Up to 4 Years
A Phase I trial of a vector gene transfer to treat advanced wet age-related macular degeneration demonstrated reproducible, sustained transgene of two therapeutic proteins that block neovascularization expression for at least 2.5 years.1
The study evaluated the lentiviral Equine Infectious Anemia Virus (EIAV) vector RetinoStat (Oxford BioMedica, Oxford, U.K.), engineered to deliver the therapeutic genes endostatin and angiostatin, each of which blocks the neovascularization characteristic of progressing neovascular AMD. Twenty-one study participants received a subretinal injection of either 2.4 × 105 TU or 8.0 × 105 TU of the viral vector in one eye. Previous study results reported the vector gene had a favorable safety and tolerability profile.
The study patients had highly fibrotic retinas and had become non-responsive to anti-VEGF therapy after having a history of response. The study showed that therapeutic gene expression, a secondary endpoint of the trial, was dose-dependent and maintained for 2.5 years in eight subjects and more than four years in two.
Mean levels of endostatin and angiostatin, measured in the aqueous humor, peaked between weeks 12 and 24 at 57 to 81 ng/mL for endostatin and 15 to 27 ng/mL for angiostatin, and remained stable through the last measurement at week 48. Long-term follow-up demonstrated the longer durations for expression.
The study reported no dose-limiting toxicities and little or no ocular inflammation. The investigators did report one procedure-related serious adverse event, a macular hole, which resolved.
“Despite an apparent reduction in fluorescein angiographic leakage that broadly correlated with the expression levels in the majority of patients, only one subject showed convincing evidence of anti-permeability activity in these late-stage patients,” said the investigators, led by Peter A. Campochiaro, MD, of the Wilmer Eye Institute at Johns Hopkins University in Baltimore.
Patients who received the 8.0 × 105 TU injection demonstrated no significant change in mean lesion size.
“These data demonstrate that EIAV vectors provide a safe platform with robust and sustained transgene expression for ocular gene therapy,” Dr. Campochiaro and co-authors said.
Other study investigators were Andreas K. Lauer of the Oregon Health Sciences Center at the University of Oregon and Elliott H. Sohn, MD, of the University of Iowa.
Dr. Lauer disclosed he is a consultant for Oxford BioMedica. Six co-authors are either former or current employees of BioMedica. Drs. Campochiaro and Sohn and co-author Tahreem A. Mir, MD, had no conflicts to disclose.
REFERENCE
1. Campochiaro PA, Lauer AK, Sohn EH, et al. Lentiviral vector gene transfer of endostatin/angiostatin for macular degeneration (GEM) study. Hum Gene Ther. 2016 Sept. 26. Epub ahead of print.



