Retinal vein occlusion is the most common retinal vascular disorder after diabetic retinopathy, affecting 1 to 2 percent of individuals older than 40 years1,2 and an estimated 16 million people worldwide.3 In patients with RVO, macular edema is the most common cause of decreased vision.4-6
 |
Industry-sponsored Phase III clinical trials have demonstrated the efficacy of monthly intravitreal ranibizumab7 (Lucentis, Roche/Genentech) and monthly aflibercept8,9 (Eylea, Regeneron) therapy for the treatment of macular edema associated with central retinal vein occlusion (CRVO). Further, case reports and small clinical trials demonstrated favorable visual outcomes following intravitreal bevacizumab (Avastin, Roche/Genentech) therapy for CRVO-associated macular edema.10-14
Ranibizumab and bevacizumab inhibit all isoforms of vascular endothelial growth factor A, and both have demonstrated similar safety and efficacy in the treatment of age-related macular degeneration15 and diabetic macular edema.16 Aflibercept, a fusion protein of key domains from both VEGF receptor 1 and VEGF receptor 2, inhibits not only all VEGF-A isoforms, but also VEGF-B and placental growth factor.17 In addition to its broader mechanism of action, aflibercept has been reported to have a higher binding affinity than ranibizumab.15,17 Bevacizumab repackaged at compounding pharmacies into syringes for intravitreal injection is much less costly than aflibercept or ranibizumab.
How SCORE2 Was Designed
The Study of COmparative Treatments for REtinal Vein Occlusion 2 (SCORE2)18-20 was designed to determine if bevacizumab is noninferior to aflibercept for the treatment of macular edema secondary to CRVO (Figure) or hemi-retinal vein occlusion (HRVO). In addition, SCORE2 compared monthly dosing to treat-and-extend dosing of aflibercept or bevacizumab from six to 12 months with respect to visual acuity and central retinal thickness at month 12 in participants who had a protocol-defined good response after six monthly injections of aflibercept or bevacizumab.
SCORE2 also
 |
evaluated the impact of alternative treatment strategies (a different anti-VEGF agent or intravitreal dexamethasone) in eyes that did not have a protocol-defined good response after six monthly injections of aflibercept or bevacizumab. SCORE2’s primary results (at six months) will be the focus of this article.
Main eligibility criteria for SCORE2 included:
best-corrected electronic Early Treatment Diabetic Retinopathy Study (E-ETDRS) visual acuity letter score (VALS) between 19 and 73 (approximate Snellen acuity 20/40 to 20/400);
center-involved macular edema due to CRVO or HRVO on clinical examination; and
central retinal thickness on spectral-domain optical coherence tomography, defined as central subfield thickness (CST) >300 µm if measured with a Carl Zeiss Meditec Cirrus OCT machine or >320 µm if measured with a Heidelberg Spectralis OCT machine.
Study eyes were randomized 1:1 to intravitreal bevacizumab (1.25 mg) every four weeks for six months vs. intravitreal aflibercept (2 mg) every four weeks for six months. Study visits were scheduled every four weeks for six months. The pre-specified primary outcome was change in best-corrected E-ETDRS VALS from baseline to month six, with the non-inferiority margin set at 5 letters.
The study enrolled 362 patients, of whom 180 were randomly assigned to aflibercept and 182 to bevacizumab. Participants’ mean age was 69 years, 43 percent were women, 76 percent white and 15 percent black. Mean VALS was 50 (approximate Snellen 20/100), and participants had macular edema for an average of seven months (range: zero to 104 months) before randomization. Mean CST was 666 µm, 33 percent had received prior anti-VEGF treatment, 8 percent had prior intravitreal steroid treatment and 16 percent were diagnosed with HRVO.
Visual Acuity Gains
At month six, bevacizumab was non-inferior to aflibercept based on a VALS margin of five (bevacizumab minus aflibercept mean difference=-0.14; p=0.001 for non-inferiority). Mean VALS improved from 50.3 at baseline to 69.3 at month six in the aflibercept group, and improved from 50.4 at baseline to 69.3 at month six in the bevacizumab group.
In the aflibercept
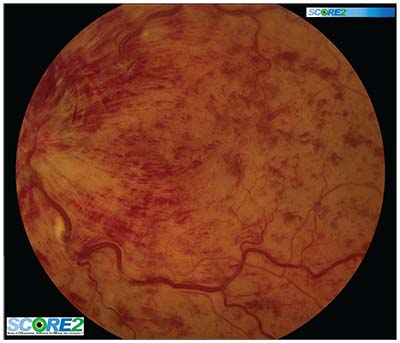 |
| Figure. A baseline image from the Study of COmparative Treatments for REtinal Vein Occlusion 2 (SCORE2) trial showing central retinal vein occlusion associated with macular edema. |
group, 65 percent of eyes had a >15-letter gain in VALS at month six vs. 61 percent in the bevacizumab group. Fewer than 2 percent in each group had a >15-letterloss in VALS at month six (aflibercept=3/175; bevacizumab=3/173). The proportion of patients who achieved a VALS of >70 (approximate Snellen of 20/40) in the study eye at month six was 58 percent in the aflibercept group and 57 percent in the bevacizumab group.
CST Reduction
Both groups demonstrated significant SD-OCT CST decreases from baseline through month six. With a baseline CST of 652 µm (SD=215 µm) in the aflibercept group, the mean decrease was 425 µm at month six. For the bevacizumab group, with a baseline CST of 678 µm (SD=233 µm), the mean decrease was 387 µm at month six.
The bevacizumab-minus-aflibercept estimate of the change from baseline treatment effect averaged over the six months was 49.3 µm in favor of aflibercept, a difference which is not statistically significant (p=0.83). In the aflibercept group, 54 percent of eyes had resolution of macular edema at month six compared with 29 percent in the bevacizumab group (p<0.001).
The Bottom Line
In sum, in SCORE2 participants with macular edema due to CRVO or HRVO, intravitreal bevacizumab was noninferior to intravitreal aflibercept with respect to visual acuity after six months of treatment, based on a non-inferiority margin of a VALS of five. Both groups demonstrated significant decreases in CST from baseline to month six, with no significant difference between the groups with respect to the magnitude of CST reduction.
Of note, the proportion of eyes that achieved resolution of macular edema at month six was significantly lower in the bevacizumab group than in the aflibercept group. While this difference was not associated with a difference between study groups in visual acuity outcomes at month six, continued follow-up of SCORE2 participants will allow us to evaluate the cumulative effect of the presence of fluid on visual acuity and on the number of injections administered in participants assigned to the treatment groups not defined by a fixed-dosing schedule.
Ongoing follow-up will also provide information regarding longer-term outcomes, including visual acuity, need for continuing treatment, development of complications of CRVO and HRVO, quality of life and morphologic outcomes. RS
REFERENCES
1. Mitchell P, Smith W, Chang A. Prevalence and associations of retinal vein occlusion in Australia. The Blue Mountains Eye Study. Arch Ophthalmol. 1996;114:1243-1247.
2. Klein R, Klein BE, Moss SE, Meuer SM. The epidemiology of retinal vein occlusion: the Beaver Dam Eye Study. Trans Am Ophthalmol Soc. 2000;98:133-141; discussion 141-143.
3. Rogers S, McIntosh RL, Cheung N, et al, and the International Disease Consortium. The prevalence of retinal vein occlusion: Pooled data from population studies from the United States, Europe, Asia and Australia. Ophthalmology. 2010;117:313-319.
4. Central Vein Occlusion Study Group. Baseline and early natural history report. The Central Vein Occlusion Study. Arch Ophthalmol. 1993;111:1087-1095.
5. Central Vein Occlusion Study Group. A randomized clinical trial of early panretinal photocoagulation for ischemic central vein occlusion. The Central Vein Occlusion Study Group N report. Ophthalmology 1995;102:1434-1444.
6. Central Vein Occlusion Study Group. Natural history and clinical management of central retinal vein occlusion. The Central Vein Occlusion Study Group. Arch Ophthalmol. 1997;115:486-491.
7. Brown DM, Campochiaro PA, Singh RP, et al, CRUISE Investigators. Ranibizumab for macular edema following central retinal vein occlusion: six-month primary end point results of a phase III study. Ophthalmology. 2010;117:1124-1133.
8. Boyer D, Heier J, Brown DM, Clark WL, et al. Vascular endothelial growth factor trap-eye for macular edema secondary to central retinal vein occlusion. Six-month results of the Phase 3 COPERNICUS Study. Ophthalmology. 2012;119:1024-1032.
9. Holz FG, Roider J, Ogura Y, et al. VEGF Trap-Eye for macular oedema secondary to central retinal vein occlusion: 6-month results of the phase 3 GALILEO Study. Br J Ophthalmol. 2013;97:278-284.
10. Wu L, Arevalo JF, Berrocal MH, et al. Comparison of two doses of intravitreal bevacizumab as primary treatment for macular edema secondary to central retinal vein occlusion. Results of the Pan American Collaborative Retina Study Group at 24 months. Retina. 2010;30:1002-1011.
11. Beutel J, Ziemssen F, Lϋke M, Partsch M, Bartz-Schmidt KU, Bevacizumab Study Group, Gelisken F. Intravitreal bevacizumab treatment of macular edema in central retinal vein occlusion: one-year results. Int Ophthalmol. 2010;30:15-22.
12. Priglinger SG, Wolf AH, Kreutzer TC, et al. Intravitreal bevacizumab injections for treatment of central retinal vein occlusion. Six-month results of a prospective trial. Retina. 2007;27:1004-1012.
13. Prager F, Michels S, Kriechbaum K, et al. Intravitreal bevacizumab (Avastin) for macular oedema secondary to retinal vein occlusion: 12-month results of a prospective clinical trial. Br J Ophthalmol. 2009;93:452-456.
14. Kriechbaum K, Michels S, Prager F, et al. Intravitreal Avastin for macular oedema secondary to retinal vein occlusion: a prospective study. Br J Ophthalmol. 2008;92:518-522.
15. The CATT Research Group. Ranibizumab and bevacizumab for neovascular age-related macular degeneration. N Engl J Med. 2011;364:1897-1908.
16. Diabetic Retinopathy Clinical Trial Network. Aflibercept, bevacizumab, or ranibizumab for diabetic macular edema. N Engl J Med. 2015;372:1193–1203.
17. Stewart MW. Aflibercept (VEGF Trap-eye): the newest anti-VEGF drug. Br J Ophthalmol 2012;96:1157-1158.
18. Scott IU, VanVeldhuisen PC, Ip MS, et al, for the SCORE Investigator Group. SCORE2 Report 2: Study design and baseline characteristics. Ophthalmology. 2017;124:245-256.
19. Scott IU, VanVeldhuisen PC, Ip MS, et al, for the SCORE2 Investigator Group. Effect of bevacizumab vs aflibercept on visual acuity among patients with macular edema due to central retinal vein occlusion. The SCORE2 randomized clinical trial. JAMA 2017:317:2072-2087.
20. Scott IU, VanVeldhuisen PC, Ip MS, et al, for the SCORE2 Investigator Group. SCORE2 Study Report 4. Baseline factors associated with 6-month visual acuity and retinal thickness outcomes in patients with macular edema secondary to central retinal vein occlusion or hemiretinal vein occlusion. JAMA Ophthalmol 2017;135:639-649



