 |
Bios Dr. Kasetty is a third-year resident at Henry Ford Hospital in Detroit. |
For eyes without capsular support, a number of solutions are available including anterior chamber intraocular lenses, iris-fixated or sutured IOLs, and scleral-fixated intraocular lenses.1 Once the realm of cataract surgeons, dislocated IOLs are often better suited to a posterior-segment approach, especially if they’re displaced into the vitreous cavity.
Shin Yamane, MD, and colleagues popularized an anterior-segment method using needle docking of haptics for scleral fixation.2 Jonathan Prenner, MD, further modified this technique for a posterior-segment surgeon. George Williams, MD, later substituted needles for 25-gauge cannulas, greatly simplifying the most tedious step.3 Mark Walsh, MD, PhD, then published a description utilizing 27-g cannulas resulting in tighter haptic clearance. 4
Following this evolution of techniques, we have modified a few minor steps to accommodate our own individual surgical preferences. The following highlight a few key components that can aid in the adoption of this complex yet elegant solution to the loss of capsular support.
Adjust the cannula position
Cannulas should be positioned 1.75 to 2 mm posterior to the limbus centered on the visual axis. A vertical orientation is preferred because the white-to-white distance is shorter than horizontal placement, resulting in reduced stretching forces on the haptics. However, rotation by up to one clock hour or so off the vertical axis may sometimes help to facilitate hand position around the nose (especially in a right eye) or to avoid areas with considerations such as a bleb (Figure 1).
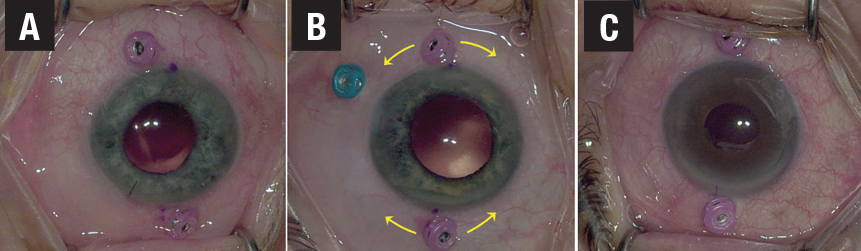 |
| Figure 1. Cannulas are placed 1.75 to 2 mm posterior to the limbus, 180° apart and centered vertically on the visual axis. B) Cannulas can be placed clockwise or counterclockwise (arrows) a few degrees from vertical to avoid the nose or a bleb. A) and C) show examples of cannulas rotated from the vertical axis counterclockwise and clockwise, respectively. |
Use a Kuglin hook
We prefer to always fixate the superior haptic first because this allows for an optimal view even through a small pupil. Drop the leading haptic through the pupil into the vitreous cavity and leave the trailing haptic in the corneal wound to hold it in place. Then with your left hand gently nudge the trailing haptic into the eye with a Kuglin hook through a paracentesis until the instrument suspends it over the pupillary axis (Figure 2). Next, use the forceps in your right hand to grasp the haptic tip.
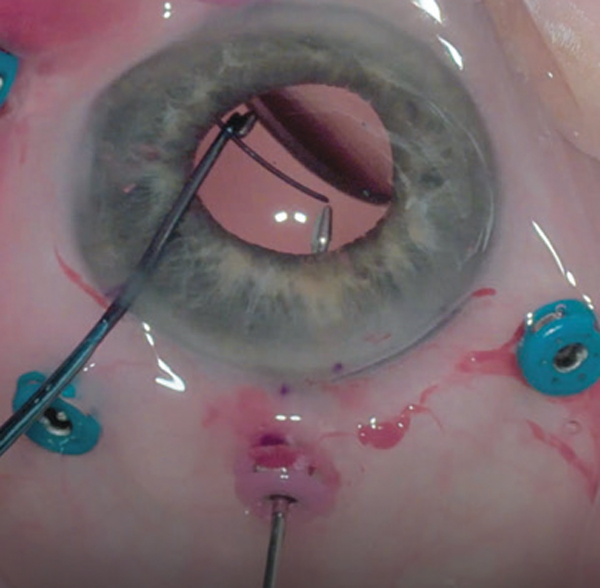 |
| Figure 2. Cannulas are placed 1.75 to 2 mm posterior to the limbus, 180° apart and centered vertically on the visual axis. B) Cannulas can be placed clockwise or counterclockwise (arrows) a few degrees from vertical to avoid the nose or a bleb. A) and C) show examples of cannulas rotated from the vertical axis counterclockwise and clockwise, respectively. |
Using the Kuglin hook has two key advantages over a more-often-used second forceps: it’s autoclaved between cases and thus doesn’t add to surgical case cost; and it won’t crush or damage the haptic while holding it in place.
Using two forceps on the same haptic for a handshake maneuver requires differential force from each hand (firm for the tip, light to none for manipulation) and takes a conscious effort. An error can pinch the haptic, causing permanent damage and requiring immediate explantation.Target the very tip when grasping the haptic with the 27-g MAXgrip forceps (Alcon). Frequently, a right or oblique angle between the shaft of the forceps and the haptic is initially created. This angle must be straightened to facilitate removal through the sclerotomy (Figure 3).
Straighten the haptic
Adjusting this angle requires a second instrument (a Kuglin hook in the AC or a light pipe in the vitreous cavity) and gentle adjustment of the grip on the forceps. If the grip is too tight, the haptic won’t rotate. If the grip is too loose, the haptic will drop from the forceps. This also serves to verify an adequate hold on the haptic before the critical externalization step.
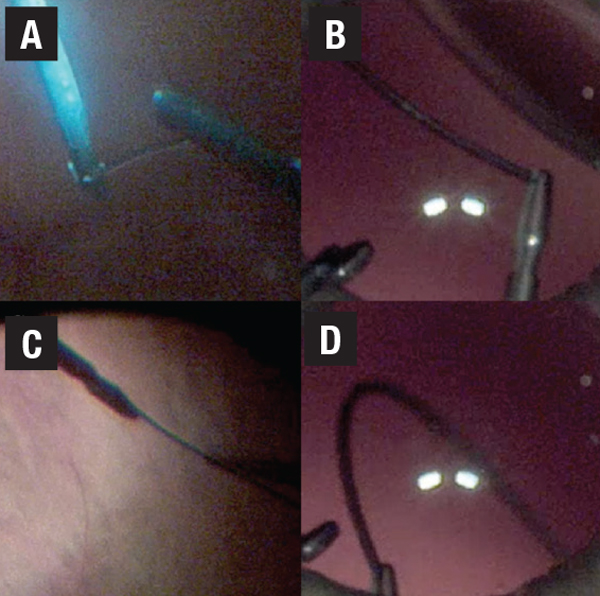 |
| Figure 3. A) and B) show a perpendicular angle between the haptic and forceps that commonly occurs when first gripping the haptic. It should be straightened before attempting to externalize the haptic. C) and D) show appropriate linear orientation of the haptic-forceps complex. |
It’s much better to drop a haptic and re-adjust it while all the instruments are in the eye rather than inadvertently releasing it during haptic externalization when the cannula is no longer in place and the forceps are exiting.
Bottom line
Scleral fixation of IOLs is frequently best approached using vitreoretinal surgical techniques. We describe augmentation of existing methods, which facilitate externalization of the haptics through 27-g sclerotomies. RS
REFERENCES
1. Holt DG, Young J, Stagg B, Ambati BK. Anterior chamber intraocular lens, sutured posterior chamber intraocular lens, or glued intraocular lens: where do we stand? Curr Opin Ophthalmol. 2012;23:62-67.
2. Yamane S, Sato S, Maruyama-Inoue M, Kadonosono K. Flanged intrascleral intraocular lens fixation with double-needle technique. Ophthalmology. 2017;124:1136-1142.
3. Abbey AM, Hussain RM, Shah AR, Faia LJ, Wolfe JD, Williams GA. Sutureless scleral fixation of intraocular lenses: Outcomes of two approaches. The 2014 Yasuo Tano Memorial Lecture. Graefes Arch Clin Exp Ophthalmol. 2015;253:1-5.
4. Walsh MK. Sutureless trocar-cannula-based transconjunctival flanged intrascleral intraocular lens fixation. Retina. 2017;37:2191-2194.




