| UPDATE ON DRUG DELIVERY |
Take-home Points
|
 |
|
Bio Dr. Kiernan is a vitreoretinal surgeon with The Eye DISCLOSURES: Dr. Kiernan is a speaker and consultant for, and receives grant support from EyePoint Pharmaceuticals. |
Noninfectious uveitis affecting the posterior segment remains one of the most confounding diseases that retina specialists treat, due in large part to the heterogeneous presentations and responses to therapy. Innovations in uveitis treatment have given clinicians more options, and the adoption of office-based therapies, such as the fluocinolone acetonide intravitreal implant 0.18 mg (Yutiq, EyePoint Pharmaceuticals) and the intravitreal dexamethasone implant 0.7 mg (Ozurdex, Allergan/AbbVie), have eased the burden on patients and decreased the risks associated with surgery.
Innovations in implant technology and sustained-release drug formulations don’t stop there. Surgical implant technology is making its way into treatments for neovascular age-related macular degeneration. These wet AMD drug candidates have followed the framework for sustained-release drug therapy that investigators first explored in the uveitis treatment pipeline.
A new concept for uveitis therapy emerges
Retina and uveitis specialists have used implant therapies that slowly elute drug to reduce treatment burden since 2008, when the fluocinolone acetonide intraocular implant 0.59 mg (Retisert, Bausch + Lomb) was shown to be safe and effective for noninfectious uveitis of the posterior segment (NIU-PS).1 David G. Callanan, MD, and colleagues demonstrated the safety and efficacy of Retisert, which is surgically implanted, in 2008.1
|
|
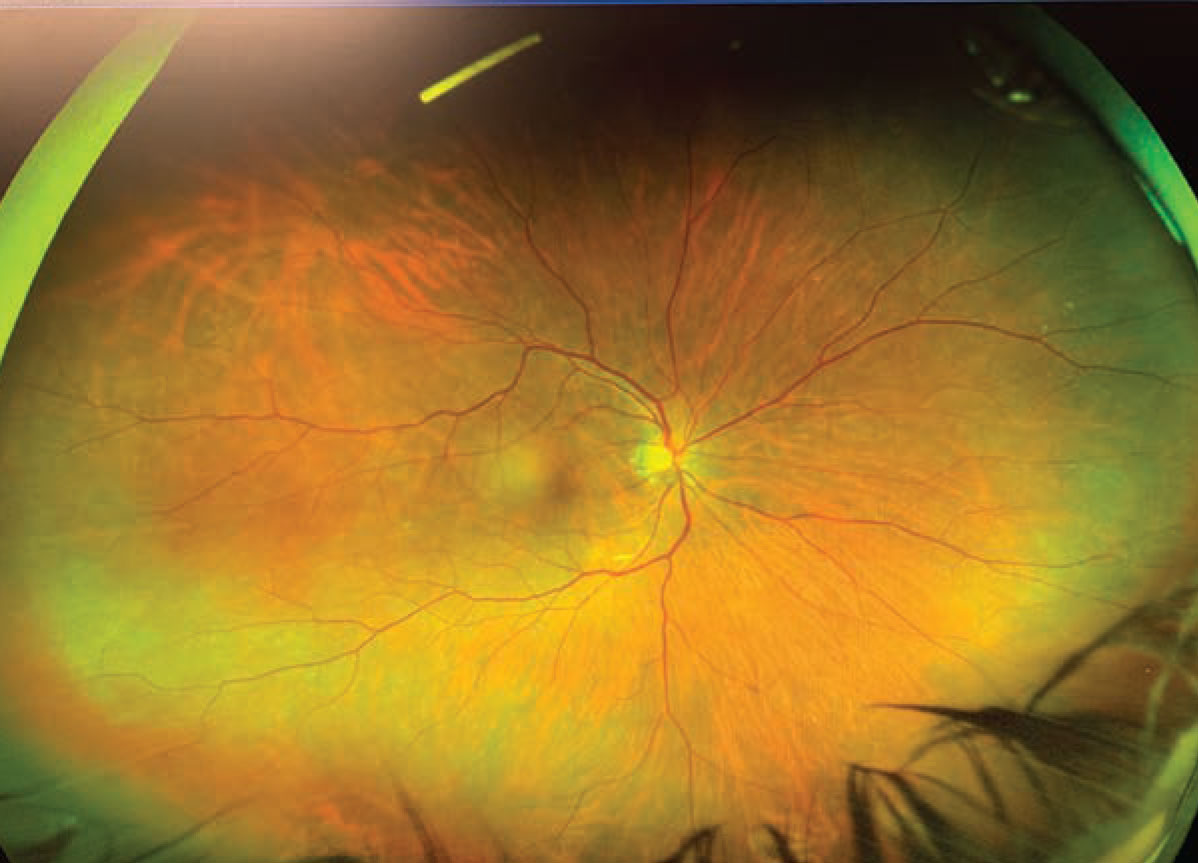
The fluocinolone acetonide intravitreal implant 0.18 mg (Yutiq, EyePoint Pharmaceuticals) can be observed at the 12 o’clock position in the posterior segment. |
In their study, they implanted Retisert at one of two doses (0.59 or 2.1 mg) in 278 patients and compared results at one, two and three years to patients who received no implant. As might be predicted with a steroid implant, patients in the treatment arms had higher rates of intraocular pressure-lowering surgery and cataract
surgery. Still, reduction in recurrence rates at all three were statistically significant. The rates of recurrence were similar among both treatment arms, and the lower dose was eventually submitted for Food and Drug Administration approval.
One major barrier to treatment with Retisert is the surgery required to implant the device. Some patients with NIU-PS may not be suited for surgery, and others may not want to accept the risks associated with it. Starting in 2010, clinicians and patients had a new steroid-based option for the treatment of uveitis that didn’t require surgery.
Dexamethasone implant
Candidates in the pipeline The pipeline has a number of promising candidates that could extend treatment intervals for patients with wet age-related macular degeneration. Three of them are:
|
A single, multicenter, randomized study of 153 patients with NIU-PS assessed the safety and efficacy of Ozurdex.2 Vitreous haze (VH) scores were examined at eight weeks in patients who received Ozurdex or sham. At that time point, 47 percent of patients in the treatment group had VH scores of 0 (i.e., no uveitis) compared with 12 percent of patients in the sham group. The difference was statistically significant.
Ozurdex has a treatment effect of three to four months, which may be appropriate for some patients with NIU-PS. Still, clinicians and patients alike may find a longer treatment interval desirable.
Extended-duration, nonsurgical option
Clinicians seeking an extended-duration, nonsurgical therapy that lasts longer than Ozurdex may turn to Yutiq, which is administered in a clinical setting and remains in the posterior segment (Figure). The three-year results of a pivotal Phase III trial assessing the safety and efficacy of Yutiq for the treatment of NIU-PS found that at month 36, patients who received Yutiq had significantly fewer uveitis recurrences compared with those who received sham injection, plus standard therapies as needed (66 vs. 98 percent, p < 0.001).3
Time to first recurrence of uveitis was 657 days in the Yutiq arm compared with 71 days in the sham arm (p < 0.001), and the mean number of recurrences in the Yutiq arm (1.7) was significantly lower than in the sham (5.3; p < 0.001). Among patients who received Yutiq, 58 percent received adjunctive treatment during the 36-month study period compared with 98 percent of sham-treated patients.
The rate of IOP-lowering surgery in the sham-treated group (12 percent) was double that of the Yutiq group (6 percent). Rates of cataract surgery were higher in the Yutiq group (74 percent) than the sham group (24 percent).
Use of Yutiq in patients with NIU-PS could potentially reduce treatment burden by obviating the need for surgical administration and reducing the frequency of adjunctive anti-inflammatory therapy, both of which may be associated with other therapies. In my experience, Yutiq has been effective at treating NIU-PS while reducing treatment burden associated.
Implants in wet AMD
The evolution of treatments for NIU-PS—from a surgically implanted device to a short-term in-office implant to a long-term in-office implant—demonstrates how innovators develop solutions that address
real-world patient concerns and help maintain therapeutic drug levels over a longer duration. As wet AMD therapies move toward extended duration and surgical solutions, we can observe the continuance of this pattern of need-based improvement.

|
The Phase III Archway trial assessed the safety and efficacy of the Port Delivery System with ranibizumab (PDS, Genentech/Roche) in patients with wet AMD. PDS is a surgically implanted device that slowly releases a customized formulation of ranibizumab. The PDS can be refilled during a process known as a refill-exchange. During a refill-exchange, a syringe clears any unused portion of drug from the implant and refills the implant with a new batch of customized ranibizumab.
The Archway investigators found that the PDS with ranibizumab was noninferior to monthly ranibizumab injections.4 As of February, about 80 percent of Archway patients reached 72 weeks.4 Among those patients, the average number of injections in the PDS arm and the monthly ranibizumab arm was 3.9 and 19.5, respectively. These data suggest that the PDS may be an option for extending treatment duration in patients with wet AMD and reducing injection burden.
The similarities between Retisert and the PDS could foretell the future of extended-duration treatment for wet AMD. In a sense, their raisons d’être are similar. They were designed to address real-world issues in a chronic disease, in patient populations with variable responses to therapy who may require frequent treatment. Looking at the wet AMD pipeline, we can anticipate similar progress in long-term therapy, from surgically implanted drug reservoirs to simpler in-office treatments.
Bottom line
Office-based therapies that extend treatment intervals for patients with NIU-PS offer the opportunity to reduce barriers to treatment and increase quality of life for patients. It remains to be seen how the lessons learned in uveitis therapy will also apply to wet AMD therapy as technology in that arena advances. RS
REFERENCES
1. Callanan DG, Jaffe GJ, Martin DF, Pearson PA, Comstock TL. Treatment of posterior uveitis with a fluocinolone acetonide implant: three-year clinical trial results. Arch Ophthalmol. 2008;126:1191-1201.
2. Ozurdex [prescribing information]. Madison, NJ: Allergan/AbbVie; 2020.
3. Jaffe GJ, Pavesio CE, Study Investigators. Effect of a fluocinolone acetonide insert on recurrence rates in noninfectious intermediate, posterior, or panuveitis: three-year results. Ophthalmology. 2020;127:1395-1404.
4. Regillo CD. Port Delivery System (PDS) for exudative AMD: Archway Phase 3 study results. Paper presented at: 2021 Angiogenesis, Degeneration, and Exudation Annual Meeting; February 13-14, 2021; Miami, FL.
5. Wong J. Phase 1 Study of an intravitreal axitinib hydrogel-based implant for the treatment of neovascular age-related macular degeneration (nAMD). Paper presented at: Association for Research in Vision and Ophthalmology Annual Meeting; May 1-7, 2021; Virtual.
6. Graybug vision reports preliminary topline results from Phase 2b ALTISSIMO Trial [press release]. Graybug; March 9, 2021; Redwood City, CA.



