 |
 |
The retina and the central nervous system share embryologic origin, and it’s thought that changes in retinal tissue may mirror those in the brain, especially in neurodegenerative disease states. Neurodegenerative disease is a general term that describes many nervous system disorders characterized by progressive death of neurons.
The idea that neurodegenerative diseases (ND) may have ocular manifestations was popularized in 1986 when researchers at the University of Southern California identified widespread degeneration of axons in the optic nerve in postmortem subjects with Alzheimer’s disease.1 This further promoted the notion that other neurodegenerative diseases may also have ocular manifestations.
NDs as a group are a common cause of morbidity in the older population and a major unmet medical need both in terms of diagnosis and treatment. Neurodegenerative diseases are typically associated with progressive change and decline in cognitive function, behavior, motor and other brain functions, eventually leading to dementia.2 Today, 50 million people worldwide are living with dementia and, as life expectancy continues to rise, this number is expected to triple by 2050.3
Making the diagnosis of neurodegenerative diseases
The differential diagnosis of neurodegenerative disease represents a major clinical challenge with significant overlap of symptoms despite disparate pathologies. The gold standard for the definitive diagnosis is brain pathology obtained at autopsy. However, deep clinical phenotyping with in vivo investigations such as structural and molecular brain imaging and cerebrospinal fluid (CSF) analyses can provide a probabilistic diagnosis premortem, such as “possible” or “probable” Alzheimer’s disease.4 However, these methods are costly, invasive, time consuming and are rarely performed before the onset of irreversible clinical symptoms. Therefore, there is a dire need for cheap, non-invasive, practical and precise screening tests for evaluation of people at risk for dementia. Because of the noted correlation between ocular pathology and ND, imaging of the eye represents a potentially powerful avenue for augmenting their diagnosis and treatment.
Retinal imaging technology has advanced considerably in the past few decades. Non-invasive, highly precise, in vivo evaluation of retinal tissue represents a feasible and cost-effective method for developing biomarkers in both advanced and presymptomatic patients.5 Here, we will review retinal changes and potential biomarkers identified via retinal imaging in some of the most common forms of neurodegenerative disease.
 |
Alzheimer’s disease
Alzheimer’s Disease, the most prevalent subtype of neurodegenerative disease, affects 5.8 million people in the United States alone and is the sixth leading cause of death. While the exact pathogenesis is not clear, AD is characterized by aggregation of abnormal extracellular beta-amyloid (Aß) plaques and intracellular tau neurofibrillary tangles, which may precede symptom onset by decades. However, histological and in-
vivo retinal imaging studies have revealed multiple manifestations of the disease in the retina itself. Perhaps most intriguing is the recent identification of Aß and tau deposition in postmortem retinal tissue, which appears to follow a perivascular pattern.6
In support of this pathological evidence, numerous retinal changes in AD have been reported in vivo. Mild cognitive impairment (MCI) is a term used to describe the first identifiable symptoms of dementia.
Even in this early disease state, both significant atrophy and hypertrophy of the retinal nerve fiber layer have been reported when comparing MCI patients to healthy controls.7 Increased RNFL thickness may indicate a later-MCI/early-AD process, since inflammation may cause local thickening prior to tissue loss, but the bulk of evidence suggests that subtle retinal changes occur in even the earliest stages of disease. In advanced stages, findings are more or less unanimous, indicating significant neurodegenerative processes such as thinning of the RNFL and ganglion cell layer (GCL), and loss of dendritic arborization when comparing AD patients to controls.6
Neurovascular changes in AD
Recently, researchers have turned their attention toward neurovascular changes in AD. Here, the retina provides a unique opportunity to quantify CNS microvasculature in vivo with a resolution not available elsewhere. Studies examining retinal vasculature alteration in AD can be categorized by either the structure or function of retinal vessels. Likely due to low measurement sensitivity, studies extracting vascular measures from fundus imaging have reported conflicting findings when comparing venular and arteriolar caliber, tortuosity and fractal dimension between AD and controls.8,9
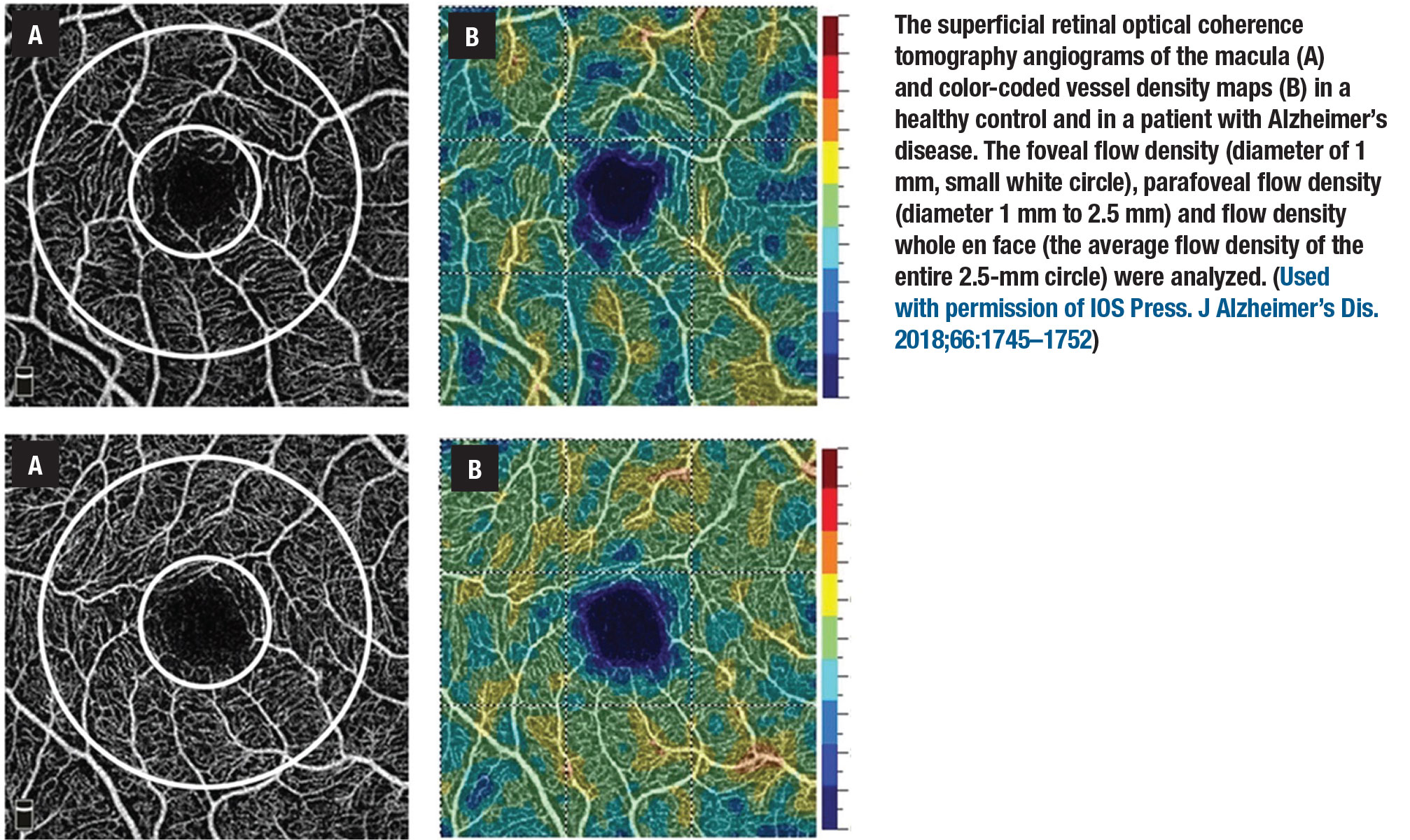
However, there seems to be a trend toward reduced vascular function when comparing AD to controls, with increased arteriolar and venular oxygen saturation, reduced blood flow and speed, and exaggerated and prolonged neurovascular coupling.10-12 Recently, optical coherence tomography angiography has become a preferred measurement of retinal vasculature, with improved resolution and sensitivity over other imaging modalities.13 Studies using OCTA have reported reduced vascular density, enlargement of the foveal avascular zone and reduced flow rates when comparing AD patients to controls.14,15
Huntington’s disease
Huntington’s disease or Huntington’s chorea is a heritable, fully penetrant and fatal ND characterized by cognitive, motor and psychiatric disturbance due to
atrophy of the basal ganglia and cerebral cortex. In western populations, its prevalence is 10.6 to 13.7 per 100,000. Unlike most neurodegenerative diseases, the pathology and cause of HD are relatively well understood—an autosomal dominant mutation in the huntingtin (HTT) gene.16 Visual dysfunction and perceptual disturbance in HD are well documented and have been reviewed thoroughly elsewhere.17
However, retinal imaging studies in HD are limited. To the best of our knowledge, only four groups have reported on them over the last decade. These studies found reduced temporal pRNFL thickness; reduced macular RNFL, GCL, inner plexiform layer (IPL), inner nuclear layer (INL), outer plexiform layer (OPL) and choroidal thickness; and no significant difference between total macular volume and global pRNFL between HD patients and controls.18-21
In addition, pRNFL and macular thicknesses were found to negatively correlate with disease duration and the Unified HD Scale Motor Score in HD patients.18,20,21
 |
Parkinson’s disease
Parkinson’s disease is one of the more common neurodegenerative diseases in the developed world, with a prevalence of 0.3 percent and an estimated incidence of eight to 18 out of 100,000.22 Disability in PD is thought to result from dysregulation and degeneration of dopaminergic neurons in the basal ganglia, accompanied by reduction of the catecholamine neurotransmitter dopamine.23 PD is a multi-system disorder characterized by both motor symptoms, such as tremor, bradykinesia and rigidity, as well as cognitive decline, hyposmia, autonomic failure and visual disturbance.22
Visual disturbance in PD ranges from reduced visual acuity to complex hallucinations, symptoms that are not confined to the retina, involving posterior cortical regions.22 However, several reports have demonstrated significant changes in retinal structure in PD. Reduced peripapillary RNFL thickness has been found globally in PD patients compared to controls.24 Reduced total macular thickness has also been observed in PD patients with more significant thinning demonstrated in the inner retinal layers.24 In addition to observed group differences in retinal volume loss, RNFL thickness has been reported to correlate with disease progression, as well as visual function.24
Researchers in Spain were able to confirm these findings longitudinally, reporting higher rates of RNFL thinning and reduction in macular thickness at five-year follow-up.25 Researchers in South Korea found that macular thickness was significantly correlated with dopamine transporter uptake density in the substantia nigra, indicating that retinal thinning was associated with dopaminergic neuronal loss in the basal ganglia.26
Emerging imaging modalities
While OCT has been the mainstay for identifying many retinal changes found in neurodegenerative diseases, a number of emerging technologies hold additional promise. In addition to OCTA, three additional technologies are at much earlier stages of investigation, but have already begun to yield insights into AD.
• Fundus autofluorescence. This imaging method is designed to capture the intrinsically fluorescent features of retinal tissue. For example, as RPE cells phagocytize outer segments, lipofuscin accumulates in the cells. Lipofuscin can fluoresce when stimulated by light from a broad spectral range spanning from 500 to 800 nm. Excess lipofuscin accumulation in surviving RPE will appear as hyper-autofluorescence, whereas RPE atrophy and loss will result in
hypo-autofluorescence. Other retinal features may also have autofluorescence patterns that are in the early stages of research. For example, beta amyloid accumulation may also be associated with autofluorescence that has yet to be clearly described.
• Fluorescence lifetime imaging ophthalmoscopy. FLIO is a new autofluorescence-based system that utilizes the FAF signal but measures the FAF signal duration or lifetime. The concept of measuring fluorescence lifetime decay in theory should provide more sensitivity and specificity for detecting pathological changes. FLIO has been used to describe early changes in macular telangiectasia type 2 (MacTel) and age-related macular degeneration as well as other degenerative diseases, which may impact macular pigments.27-30
• Dynamic vessel analysis. DVA is a system that evaluates real-time morphological changes in the retinal microvasculature in response to stimuli presented in the form of flickering lights. Eyes of healthy controls have been shown to demonstrate a characteristic response curve, with primary vasodilation and secondary vasoconstriction when presented with the stimulus. AD patients have been shown to not only have a decreased reaction amplitude but a reduction in arterial dilation as well.31 This change has been described as a downstream effect of neurovascular decoupling in the progression of both vessel and nerve disease.
 |
Limitations of imaging studies
As with any emerging field, there are several limitations to the conclusions gleaned from these imaging studies. First and foremost, there is no direct causation yet established between Alzheimer’s disease severity, duration or progression with RNFL thickness or microvascular density. This is related to the question of receiving an AD diagnosis in and of itself and the fact that the site of disease—the brain—is rather inaccessible until postmortem in most cases.
Moreover, diseases such as AMD, glaucoma, vascular diseases including diabetes and hypertension, and other confounders that affect the macula are prevalent in aging populations and may be overestimating the contribution of AD to these measurements.
The impact of medications used to treat these diseases themselves can have effects on the measurements of microvascular morphology, further confounding conclusions. Ultimately, longitudinal studies using well-developed and established imaging modalities will need to be conducted to tease apart the multifactorial contributions to retinal changes that have been identified in neurodegenerative disease.
 |
Bottom line
An ounce of prevention is worth a pound of cure. By monitoring thinning in the GCL and RNFL, watching for changes to vessel morphology and assaying vessel density, we may be able to combine different imaging modalities to determine a patient’s diagnostic likelihood for dementia. We may then begin to answer questions regarding prevention and treatment efficacies and make observations on whether retinal changes are progressive, and whether they can be reversed. In this way, noninvasive ophthalmic imaging is likely to continue to develop an increasing role in the diagnosis and possibly management of subjects with dementia. RS
REFERENCES
1. Hinton D, Sadun A, Blanks J, Miller C. Optic-nerve degeneration in Alzheimer’s disease. N Engl J Med, 1986;315:485-487.
2. Erkkinen M, Kim MO, Geschwind M. Clinical neurology and epidemiology of the major neurodegenerative diseases. Cold Spring Harb Perspect Biol. 2018;10: a033118.
3. Alzheimer’s Disease International. World Alzheimer Report 2018, The state of the art of dementia research: New frontiers. https://www.alz.co.uk/research/world-report-2018 Published September 2018. Accessed October 29, 2019.
 |
4. Elahi F, Miller B. A clinicopathological approach to the diagnosis of dementia. Nat Rev Neurol. 2017;13:457-476.
5. Cheung CY, Ikram MK, Chen C, Wong, TY. Imaging retina to study dementia and stroke. Prog Retin Eye Res. 2017;57:89-107.
6. Hart N, Koronyo Y, Black K, Koronyo-Hamaoui M. Ocular indicators of Alzheimer’s: Exploring disease in the retina. Acta Neuropathologica. 2016;132:767-787.
7. Srinivasan S, Efron N. Optical coherence tomography in the investigation of systemic neurologic disease. Clin and Exp Optom. 2017;102:309-319.
8. Cheung CK, Ong YT, Ikram MK, et al. Microvascular network alterations in the retina of patients with Alzheimer’s disease. Alzheimers Dement. 2014;10:135-142.
9. Jung NY, Han J, Ong YT, et al. Retinal microvasculature changes in amyloid-negative subcortical vascular cognitive impairment compared to amyloid-positive Alzheimer’s disease. J Neurol Sci. 2019;396:94-101.
10. Frost S, Bhuiyan A, Offerman D, et al. Modulation of retinal arteriolar central reflection by APOE genotype. Curr Alzheimers Res. 2017;14:916-923.
11. Jiang H, Liu Y, Wei Y, et al. Impaired retinal microcirculation in patients with Alzheimer’s disease. PLoS One. 2018;13:e0192154
12. Olafsdottir OB, Saevarsdottir HS, Hardarson SH, et al. Retinal oxygen metabolism in patients with mild cognitive impairment. Alzheimers Dement (Amst). 2018;10:340-345.
13. Kashani AH, Chen CL, Gahm JK, et al. Optical coherence tomography angiography: A comprehensive review of current methods and clinical applications. Prog Retin Eye Res. 2017;60:66-100.
14. Bulut M, Kurtuluş F, Gözkaya O, et al. Evaluation of optical coherence tomography angiographic findings in Alzheimer’s type dementia. Br J Ophthalmol. 2018;102: 233-237.
15. Lahme L, Esser E, Mihailovic N, et al. Evaluation of ocular perfusion in alzheimer’s disease using optical coherence tomography angiography. J Alzheimers Dis. 2018;66:1745-1752.
16. McColgan P, Tabrizi S. Huntington’s disease: A clinical review. Eur J Neurol. 2018;25:24-34.
17. Coppen EM, van der Grond J, Hart EP, Lakke EAJF, Roos RAC. The visual cortex and visual cognition in Huntington’s disease: An overview of current literature. Behav Brain Res. 2018;351:63-74.
18. Andrade D, Beato J, Monteiro A, et al. Spectral-domain optical coherence tomography as a potential biomarker in Huntington’s Disease. Mov Disord. 2016;31:377-383.
19. Gatto E, Parisi V, Persi G, et al. (2018, 12 2). Optical coherence tomography (OCT) study in Argentinean Huntington’s disease patients. Int J Neurosci. 2018;128:1157-1162.
20. Gulmez Sevim D, Unlu M, Gultekin M, Karaca C. Retinal single-layer analysis with optical coherence tomography shows inner retinal layer thinning in Huntington’s disease as a potential biomarker. Int Ophthalmol. 2019;39:611-621.
21. Kersten H, Danesh-Meyer H, Kilfoyle D, Roxburgh R. Optical coherence tomography findings in Huntington’s disease: A potential biomarker of disease progression. Neurol. 2015;262:2457-2465.
22. Archibald N. Clarke M, Mosimann U, Burn D. The retina in Parkinson’s disease. Brain. 2009;132:1128-1145.
23. Galvan A, Wichmann T. Pathophysiology of parkinsonism. Clin Neurophysiol. 2008;119:1459-1474.
24. Doustar J, Torbati T, Black K, Koronyo Y, Koronyo-Hamaoui M. Optical coherence tomography in Alzheimer’s disease and other neurodegenerative diseases. Front Neurol. 2017;8: 701.
25. Satue M, Rodrigo M, Obis J, et al. Evaluation of progressive visual dysfunction and retinal degeneration in patients with Parkinson’s disease. Invest Opthalmol Vis Sci. 2017;58:1151.
26. Ahn J, Lee JY, Kim T, et al. Retinal thinning associates with nigral dopaminergic loss in de novo Parkinson disease. Neurology. 2018;91:e1003-e1012.
27. Sadda S, Borrelli E, Fan W, et al. A pilot study of fluorescence lifetime imaging ophthalmoscopy in preclinical Alzheimer’s disease. Eye. 2019;33:1271-1279.
28. Sauer L, Andersen KM, Dysli C, Zinkernagel MS, Bernstein PS, Hammer M. Review of clinical approaches in fluorescence lifetime imaging ophthalmoscopy. J Biomed Opt. 2018;23:1-20.
29. Sauer L, Andersen K, Li B, Gensure R, Hammer M, Bernstein P. Fluorescence lifetime imaging ophthalmoscopy (FLIO) of macular pigment. Invest Ophthalmol Vis Sci. 2018;59:3094-3103
30. Jentsch S, Schweitzer D, Schmidtke KU, et al. Retinal fluorescence lifetime imaging ophthalmoscopy measures depend on the severity of Alzheimer’s disease. Acta Ophthalmologica. 2015;93:e241-e247.
31. Querques G, Borrelli E, Sacconi R, et al. Functional and morphological changes of the retinal vessels in Alzheimer’s disease and mild cognitive impairment. Sci Rep. 2019;9:63.



