| New Insights into Gene Therapy |
Take-home points
|
 |
|
Bios Dr. Nagiel is an assistant professor of ophthalmology at Children’s Hospital Los DISCLOSURES: Dr. Cao has no financial disclosures. Dr. Nagiel is a consultant for Allergan/AbbVie, Biogen, Novartis and RegenxBio. |
Voretigene neparvovec-ryzl represents the first Food and Drug Administration-approved gene therapy of its kind for the treatment of RPE65-mediated retinal dystrophy.1 This treatment is now being delivered at 10 designated ocular gene therapy treatment centers across the United States.
As with any newly approved therapy, the first few years of real-world use can provide significant insight into how well the treatment performs along with any treatment-related side effects that may not have revealed in the controlled clinical trial environment. Our group last year presented the eagerly awaited interim one-year analysis of the post-authorization safety study (PASS) at the Retina Society in Chicago, allowing us a peek into how early experiences with voretigene neparvovec-rzyl (VN, Luxturna, Spark Therapeutics) holds up against clinical trial data.2
Here we discuss those findings along with our center’s experience, and its potential implications for the future of retinal gene therapy.
PASS one-year interim analysis
This study represents a subset (37 of the 88 total patients) who received VN between March 2018 and April 2019 across the 10 designated U.S. treatment centers. It’s important to recognize that this interim analysis represents only a subset of the total study population.
The majority of treatment-emergent adverse events (TEAEs) were mild in nature and similar to those found at year 1 in the Phase III clinical trial. Serious TEAEs included one case of foveal degeneration and two cases of rhegmatogenous retinal detachment, all of which were determined to be related to the administration procedure itself.
Also reported were several cases of chorioretinal atrophy (Figure 1), a TEAE that wasn’t previously recognized during the clinical trials, having been first described by William S. Gange, MD, and colleagues.3
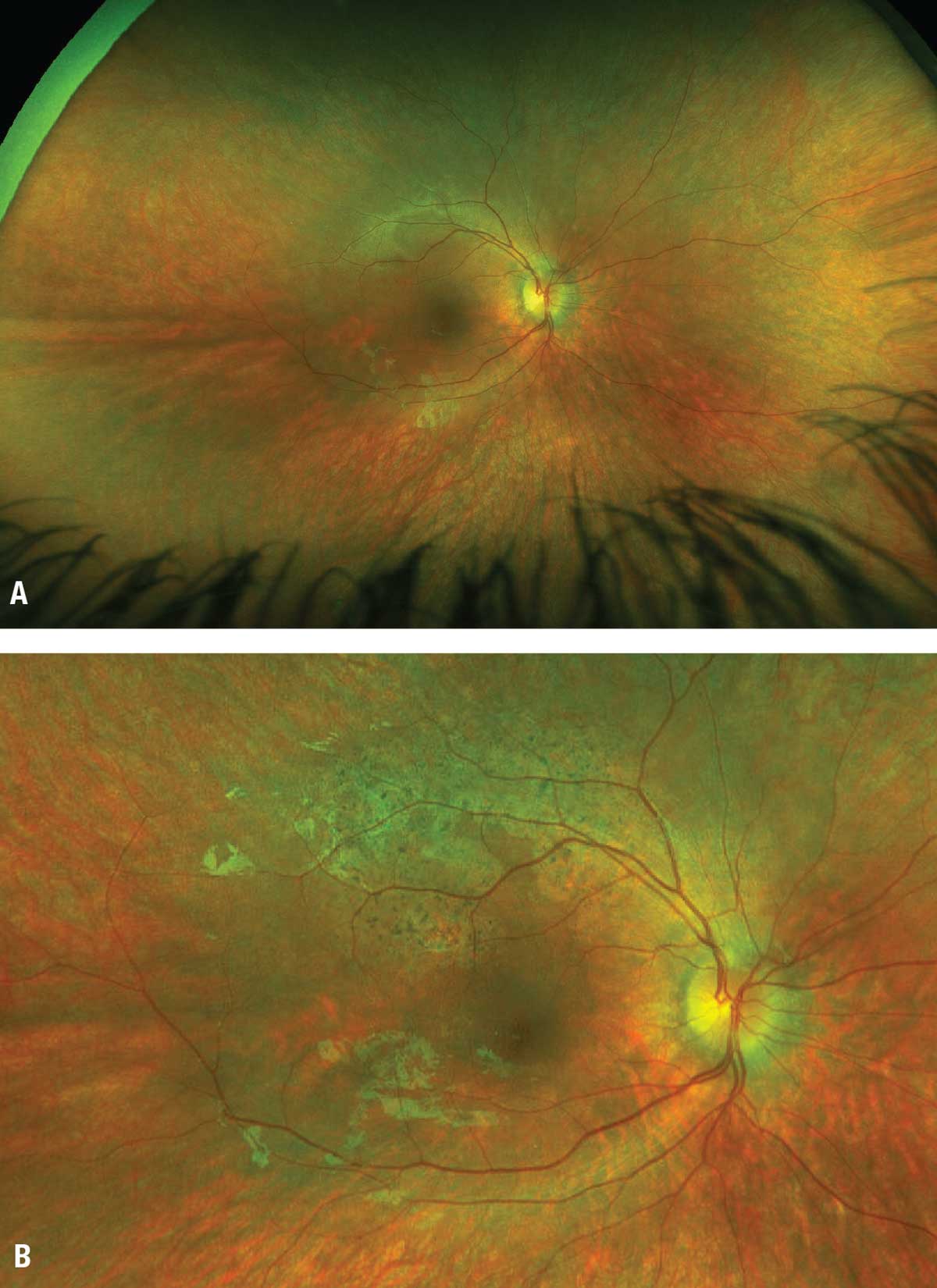 |
| Figure 1. Perifoveal atrophy in the right eye of a 6-year-old boy treated with voretigene neparvovec-rzyl at (A) baseline and (B) one year postoperatively. |
Altogether, these safety data were generally consistent with the known clinical safety profile of VN and didn’t identify any unacceptable barriers to its continued use. The one- and five-year follow-up data across the full cohort are highly anticipated to further monitor the safety and efficacy of VN from a larger overall patient population.
Surgical delivery techniques
Many of the designated ocular gene therapy centers initially delivered the vector according to the recommendations of the Luxturna surgical manual. This entailed the use of connection tubing that allowed a skilled assistant to inject the vector while the primary surgeon positioned the subretinal cannula.
However, this approach has its downsides, including that the primary surgeon can’t have full hand-eye-foot pedal control of the injection. Also, it requires a trained assistant who may not always be available.
As a result, many treatment centers have pivoted toward foot-pedal control via the silicone oil injection setup using the MicroDose Injection Kit (MedOne Surgical). Before loading the vector into the syringe, it’s essential to mobilize the syringe’s stopper using the inject/extract functions.
 |
Once the vector is loaded with the subretinal cannula attached, we then deliver the subretinal bleb at an intraocular pressure of 10 mmHg and maximum injection pressure of 10 to 16 PSI. This allows for some play in the pedal, since the max pressure level is typically not necessary to achieve slow, steady bleb propagation.
Another important adjunct is the use of intraoperative ocular coherence tomography guidance to precisely determine the location and extent of the bleb, including the ability to monitor for fovea detachment during delivery (Figure 2).
Chorioretinal atrophy
The one-year interim analysis also showed that a minority of patients undergoing treatment with VN developed chorioretinal atrophy due to etiologies that aren’t entirely understood at this time. A 2022 multicenter retrospective analysis by Dr. Gange and colleagues found a total of 18 eyes from 10 patients who underwent treatment with VN who developed progressive chorioretinal atrophy at four treatment centers across the United States.3
In this study, patients were identified as having chorioretinal atrophy if they met two conditions: if the areas of atrophy weren’t considered directly related to the subretinal cannula touchdown site; and if the atrophic areas displayed progressive enlargement over time. Eight of the 10 patients developed bilateral atrophy. The areas of atrophy were noted to be within and outside of the bleb in 10 eyes (55.5 percent), exclusively within the bleb area in seven eyes (39 percent), and exclusively outside of the bleb in one eye (5.5 percent).
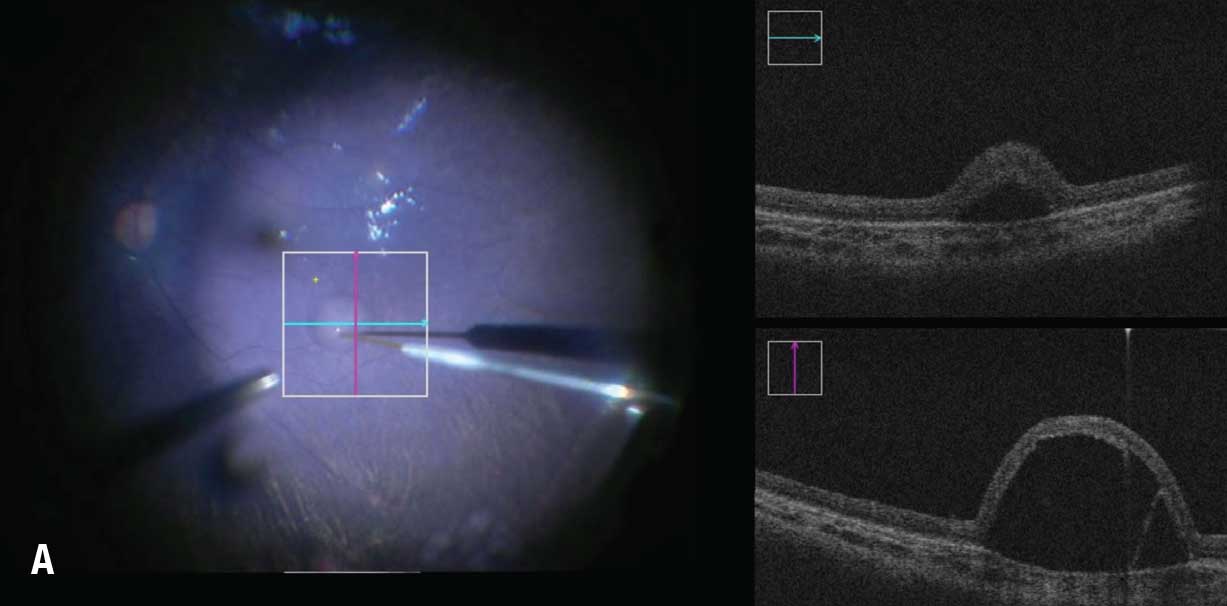 |
| Figure 2A. Subretinal delivery of voretigene neparvovec-rzyl into the right eye of a 22-month-old girl. Bleb creation using intraoperative optical coherence tomography guidance. |
While there was no statistically significant change in visual acuity, patients did show consistent improvements in full-field stimulus threshold testing and Goldmann visual fields, with a subset
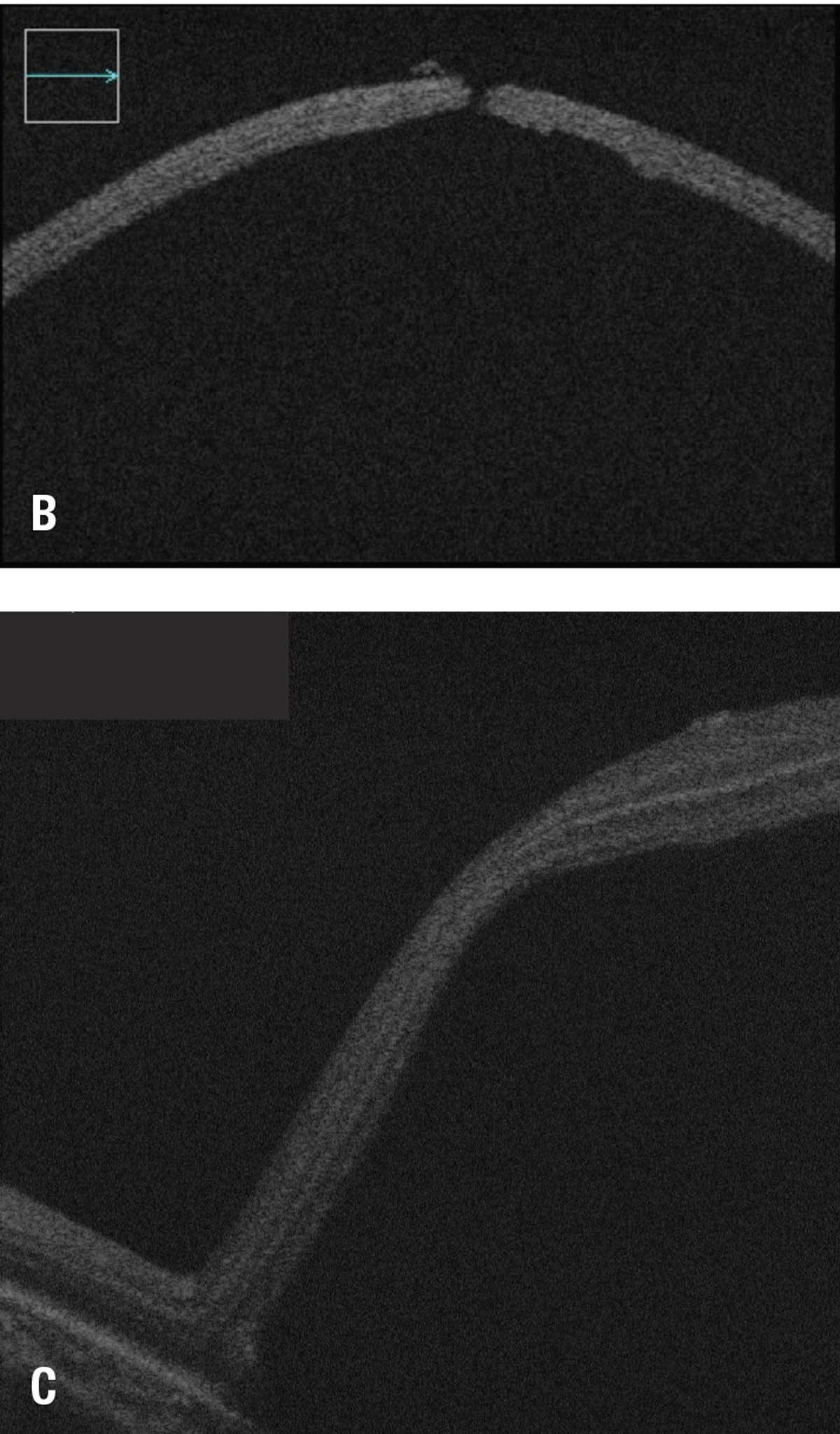 |
| Figures 2B and 2C. B) OCT cross-sectional view of the retinotomy site in same patient as figure 2A. C) Intraoperative monitoring of foveal detachment in same patient. |
demonstrating paracentral scotomas related to the atrophic changes. Studies are ongoing to identify ocular factors, surgical delivery techniques and vector-related factors that may contribute or predispose to the development of chorioretinal atrophy in patients undergoing treatment with VN.
Emerging retinal gene therapies
The early real-world success of subretinal VN delivery is encouraging as a number of other gene therapies for inherited retinal diseases continue to make their way through the pipeline. MeiraGTx/Janssen’s ongoing gene therapy trials for RPGR have also shown encouraging data that the therapy is well-tolerated and can lead to significant improvements in vision for those with X-linked retinitis pigmentosa.4 Similarly, Applied Genetic Technology Corp.’s trials for RPGR are continuing based on promising results from earlier Phase I/II trials.5
Active pivotal gene therapy studies for CNGA3 and CNGB3-linked achromatopsia hold promise to improve visual acuity and contrast sensitivity in treated patients.6,7 Several other subretinal gene therapy trials are under way or being planned, so the next few years will be an exciting time.8,9
Gene therapy beyond IRD
The promise of retinal gene therapy extends beyond the realm of treating inherited retinal diseases. RGX-314 (RegenxBio) is being developed as a subretinal gene therapy targeting vascular endothelial growth factor by delivering a gene encoding a therapeutic anti-VEGF Fab protein.
After it’s injected into the subretinal space, RGX-314 is designed to produce continuous anti-VEGF therapy. Two pivotal trials are underway comparing outcomes in patients receiving two dosages of RGX-314 with those receiving ranibizumab (ATMOSPHERE, NCT04704921)9,10 and aflibercept (ASCENT, no NCT number listed) in neovascular age-related macular degeneration.10,11
RegenxBio is also testing a similar vector for suprachoroidal delivery in the AAVIATE11 (NCT04514653) and ALTITUDE (NCT04567550)12 trials in nAMD and diabetic macular edema, respectively.11 This suprachoroidal approach has definite advantages for therapeutics, functioning as a “biofactory” by avoiding the need for vitrectomy.
Finally, another gene therapy approach is via direct intravitreal injection. ADVM-022 (Adverum) is an adeno-associated virus (AAV) vector designed to generate aflibercept. It’s being tested in the OPTIC (NCT03748784) and INFINITY (NTC04418427) trials for nAMD and DME, respectively.13 This approach is promising, but questions arose after one subject with DME developed panuveitis, vision loss and hypotony.14
If approved at some point in the future, these therapies could significantly reduce treatment burden for a variety of VEGF-mediated ischemic retinopathies, including nAMD, retinal vein occlusion and diabetic retinopathy.
Bottom line
Real-world data for VN thus far has been encouraging and has shown a safety profile largely comparable to results found in clinical trials. Most treatment-related adverse events were transient, mild and responsive to treatment. The age of gene therapy for retinal diseases is here, and its promise extends beyond just inherited retinal disease to common eye diseases with significant societal burden. RS
REFERENCES
1. Russell S, Bennett J, Wellman JA, et al. Efficacy and safety of voretigene neparvovec (AAV2-hRPE65v2) in patients with RPE65-mediated inherited retinal dystrophy: A randomised, controlled, open-label, Phase 3 trial. Lancet. 2017;390:849-860.
2. Nagiel, A, Aleman, T, Comander, J, et al. A multicenter, non-interventional, observational, post-authorization safety study of patients treated with voretigene neparvovec-rzyl gene therapy in the United States: Interim analysis at one year. Paper presented at Retina Society annual meeting; Chicago, IL; October 1, 2021.
3. Gange WS, Sisk RA, Besirli CG, et al. Perifoveal chorioretinal atrophy after subretinal voretigene neparvovec-rzyl for RPE65-mediated Leber congenital amaurosis. Ophthalmol Retina. 2022;6:58-64.
4. Michaelides M, Besirili C, Khan K, et al. AAV5-RPGR gene therapy for RPGR-associated X-linked retinitis pigmentosa: 9-month results from a Phase 1/2 clinical trial. Paper presented at EURETINA Virtual Meeting; October 3, 2020.
5. Sisk R. Clinically meaningful visual improvements demonstrated 12 months after AGTC-501 gene therapy for X-linked retinitis pigmentosa. Poster presented at American Academy of Ophthalmology 2021; New Orleans, LA; November 12-15, 2021.
6. No author available. Encouraging results from achromatopsia gene therapy trials. DBGen Ocular Genomics website. Published August 31, 2020. Available at: https://dbgen.com/en/encouraging-results-from-achromatopsia-gene-therapy-trials/.
7. Fischer MD, Michalakis S, Wilhelm B, et al. Safety and vision outcomes of subretinal gene therapy targeting cone photoreceptors in achromatopsia: A nonrandomized controlled trial. JAMA Ophthalmol. 2020;138:643-651.
8. Koulisis N, Nagiel A. Precision therapy for inherited retinal disease: At the forefront of genomic medicine. Clin Lab Med. 2020;40:189-204.
9. Ho AC. Subretinal gene therapy for exudative AMD. Paper presented at Angiogenesis, Exudation and Degeneration 2021 virtual meeting; February 13, 2021.
10. RegenxBio announces initiation of second pivotal trial in RGX-314 clinical program for the treatment of wet AMD using subretinal delivery. [press release] RegenxBio; Rockville, MD; January 10. 2022. Available at: https://regenxbio.gcs-web.com/news-releases/news-release-details/regenxbio-announces-initiation-second-pivotal-trial-rgx-314.
11. Avery RL. Two-year results from the subretinal RGX-314 gene therapy Phase 1/2a study for the treatment of nAMD and an update on suprachoroidal trials. Paper presented at Subspecialty Day: Retina, American Academy of Ophthalmology; New Orleans, LA; November 12, 2021
12. Marcus D. Suprachoroidal delivery of RGX-314 for diabetic retinopathy without CI-DME: Early results from the Phase II ALTITUDE study. Paper presented at American Society of Retina Specialists; San Antonio, TX; October 9, 2021.
13. Boyer DS. Results from a Phase 2 study of ADVM-022 intravitreal gene therapy for diabetic macular edema: The INFINITY trial. Paper presented at Subspecialty Day: Retina, American Academy of Ophthalmology; New Orleans, LA; November 12, 2021.
14. Adverum provides update on ADVM-022 and the INFINITY trial in patients with diabetic macular edema. [press release] Adverum Biotechnologies; Redwood City, CA; July 22, 2021. Available at: https://investors.adverum.com/news/news-details/2021/Adverum-Provides-Update-on-ADVM-022-and-the-INFINITY-Trial-in-Patients-with-Diabetic-Macular-Edema/default.aspx.



