With the Food and Drug Administration approval of what’s now known as Vabysmo, retina specialists have the option of an intravitreal agent for neovascular age-related macular degeneration and diabetic macular edema that targets two disease pathways and can extend treatment intervals out to four months.
The approval of faricimab-svoa—formerly just faricimab—came days after the publication of one-year results from four Phase III trials.1,2 Genentech says Vabysmo will be available in the United States “in the coming weeks.” The London analytics firm Clarivate reports that Vabysmo is projected to have more than $1 billion in annual sales.
Faricimab is what’s known as a bispecific antibody. That is, it targets two key pathways that contribute to retinal disease: vascular endothelial growth factor-A—the same VEGF complement that aflibercept (Eylea, Regeneron Pharmaceuticals) targets—and angiopoietin-2 (Ang-2).
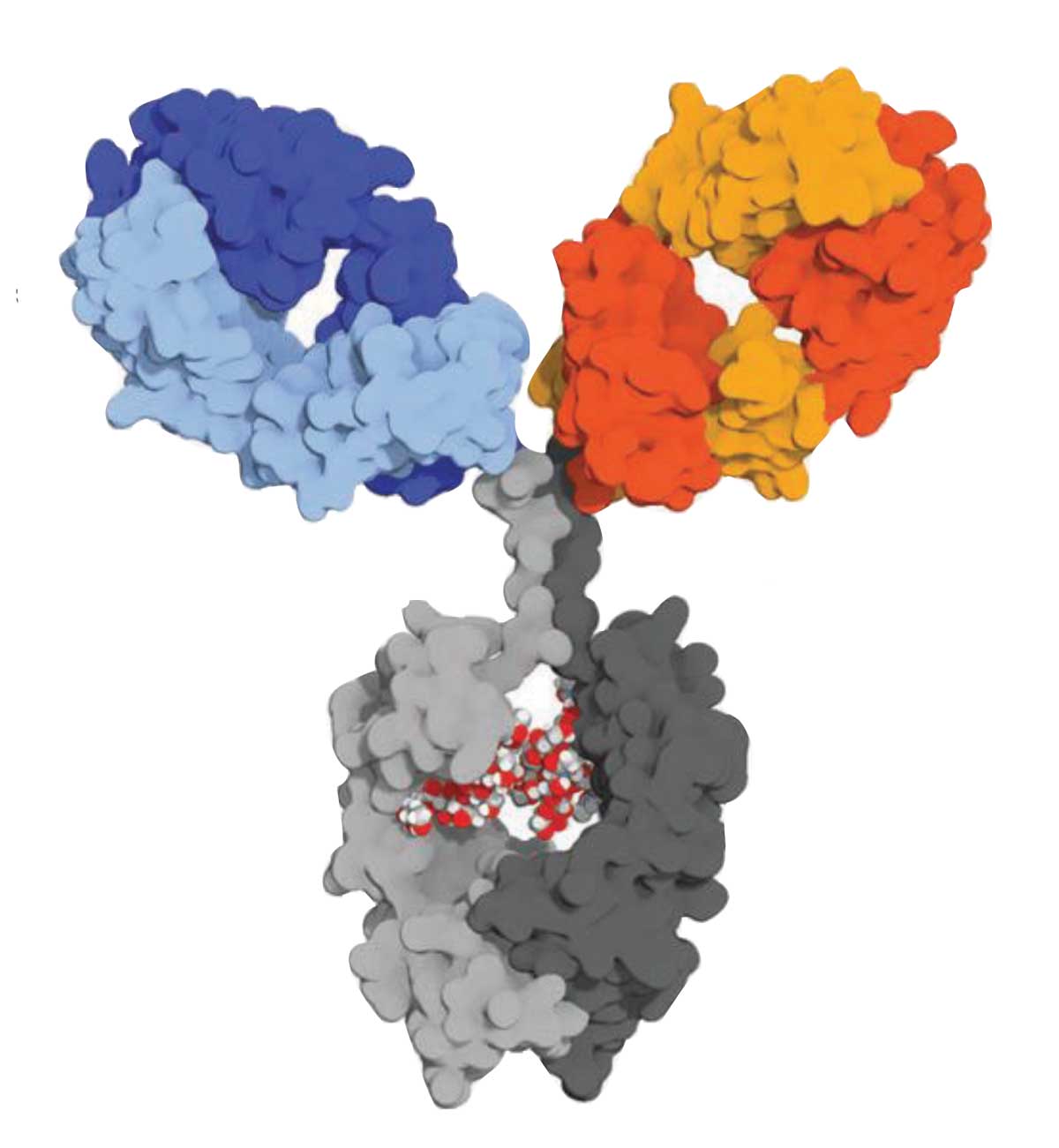 |
| Vabysmo consists of one molecule with two active arms: an anti-VEGF-A fragment antibody (Fab) (blue) and an anti-Ang-2 Fab (orange and red). The optimized fragment (gray) has no effective agent. |
Trial results
In nAMD, 45 percent of faricimab-treated patients between the TENAYA and LUCERNE trials1 received treatment every 16 weeks after four monthly loading doses, one third continued with 12-week dosing and the rest every eight-week dosing.
In DME, more than half the patients in the YOSEMITE and RHINE studies combined2 were treated every 16 weeks—51.8 percent across both studies—while 20.5 percent continued with 12-week dosing, 15.5 percent had eight-week intervals and 12 percent were on monthly dosing. The studies used two faricimab dosing intervals: up to 16 weeks after four monthly loading doses using a treat-and-extend approach; and eight-week intervals after six monthly loading doses.
Jeffrey S. Heier, MD, lead author of the TENAYA and LUCERNE results, says faricimab potentially meets the need for extended treatment duration. “The treatment burden is something that we’ve all understood for 15 years now since the first approvals of anti-VEGF beginning with pegaptanib, but then continuing with the stronger anti-VEGFs ranibizumab and aflibercept,” he says.
“But there’s always been this challenge to maintain maximum benefit with a minimum of visits to the clinic; to minimize the treatment burden while maximizing the treatment benefit,” he says. “The hope is here that we now have an agent that will enable us to extend those treatment intervals but still maintain the same benefits that we’ve seen to date and do so in a safe manner.” Dr. Heier is copresident and medical director of Ophthalmic Consultants of Boston.
Targeting Ang-2
Charles C. Wykoff, MD, PhD, lead author of the YOSEMITE and RHINE report, explains the importance of targeting Ang-2, which neutralizes the vasoprotective effects of the Ang-1 and Tie2 signaling pathway. “There’s a great deal of basic science data to support that activation of Tie2 transmembrane receptor tyrosine kinase receptor can bring additional value that’s separate from inhibition of VEGF,” he says.
“The approach now that has been validated with the Phase III trials is to inhibit angiopoietin-2, which then subsequently translates into activation of the TI2 receptor,” Dr. Wykoff adds. He’s chief medical editor of Retina Specialist, partner in Retina Consultants of Texas and deputy chair of ophthalmology at the Blanton Eye Institute, Houston Methodist Hospital.
Both Dr. Heier and Dr. Wykoff disclosed relationships with Genentech/Roche. F. Hoffmann-La Roche sponsored the trials. RS
REFERENCES
1. Heier JS, Khanani AM, Ruiz CQ, et al. Efficacy, durability, and safety of intravitreal faricimab up to every 16 weeks for neovascular age-related macular degeneration (TENAYA and LUCERNE): Two randomised, double-masked, phase 3, non-inferiority trials. Lancet. Published online January 24, 2022. doi: 10.1016/S0140-6736(22)00010-1
2. Wykoff CC, Abreau F, Adamis AP, et al. Efficacy, durability, and safety of intravitreal faricimab with extended dosing up to every 16 weeks in patients with diabetic macular oedema (YOSEMITE and RHINE): Two randomised, double-masked, phase 3 trials. Lancet. Published online January 24, 2022. doi: 10.1016/S0140-6736(22)00018-6.
In BriefRegenXbio has initiated ASCENT, the second of two Phase III pivotal trials to of its potential one-time subretinal gene therapy candidate RGX-314 for neovascular age-related macular degeneration. The primary trial endpoint is noninferiority to aflibercept based on improvement in visual acuity. The trial will enroll around 465 patients. Nanoscope Therapeutics has received Investigational New Drug (IND) clearance from the Food and Drug Administration to begin a Phase II trial of its MCO-010 ambient-light activatable optogenetic monotherapy to restore vision in Stargardt disease. Applied Genetic Technologies Corp. reports exceeding the enrollment target in the SKYLINE Phase I/II trial of AGTC-501, a recombinant adeno-associated virus vector-based gene therapy for X-linked retinitis pigmentosa (XLRP). Fourteen patients have been enrolled; the planned target enrollment was 12. The FDA has granted Fast Track Designation to 4D Molecular Therapeutics’ gene therapy candidate 4D-125 to treat inherited retinal dystrophies due to defects in the RPGR gene, including XLRP. The FDA has accepted the IND application for Ocugen to start a clinical trial of OCU400 (AAV-NR2E3), a modifier gene therapy candidate for retinitis pigmentosa. |
Study Elucidates HCQ Retinopathy Risk
In the first year of the COVID-19 pandemic, hydroxychloroquine received a lot of attention for its purported therapeutic effects for treating the disease. However, it has long been used in rheumatology, and retina specialists have been well aware of its vision-threatening side effects.
Now researchers have potentially quantified the risks for retinopathy in patients taking HCQ for systemic lupus erythematosus and other rheumatoid disease. “We found that the HCQ dose relative to body weight was the major risk factor for the development of retinopathy and there was a dose-response relationship,” April Jorge, MD, said at the American College of Rheumatology virtual meeting in November. She presented results of a case-control study of 4,899 patients who had been on HCQ for five years or longer.1
Dr. Jorge, a rheumatologist at Massachusetts General Hospital and an instructor at Harvard Medical School in Boston, said the odds of retinopathy were lowest for patients on ≤ 4 mg/kg HCQ daily, were higher in those using 5 to 6 mg/kg a day, and highest with ≥ 6 mg/kg a day.
“We also found that longer duration of use was another major risk factor contributing to retinopathy risk,” Dr. Jorge said. “For every five years of use, the risk doubled.”
Other significant risk factors were chronic kidney disease and Asian ancestry. The latter, Dr. Jorge said, came with a higher prevalence of the atypical pericentral pattern retinopathy that can be more difficult to detect.
The study evaluated a population of patients in the Kaiser Permanente Northern California system who had been on continuous HCQ therapy for at least five years between 1997 and 2020 and had regular retinopathy screenings after five years of therapy. In all, 164 had developed HCQ retinopathy—an incidence of 3.3 percent. Most cases (n=100) were mild, but 38 were moderate and 26 severe. A parafoveal pattern was noted in 131 and a pericentral pattern in 33.
“With regular screening, the majority of these cases are mild and therefore asymptomatic,” Dr. Jorge said. Patients with additional risk factors need closer monitoring and dose adjustment, she said.
“The risk of HCQ retinopathy really needs to be weighed against the benefits of this medication,” she said. RS
Dr. Jorge has no disclosures.
REFERENCE
1. Jorge A, Melles R, Conell C, et al. Risk factors for hydroxychloroquine retinopathy and its subtypes–prospective adjudication analysis of 4,899 incident users. Paper presented at virtual American College of Rheumatology Convergence 2021; November 7, 2021.



