Quality of life in medicine is a nebulous term because it means different things to different people. Hundreds of different instruments to measure quality of life have been developed. Prof. Sam Salek discussed 120 quality-of-life instruments in a compendium published almost 20 years ago.1 Hundreds more have been created since then. There is no criterion or gold standard quality-of-life instrument, explaining in part why so many quality-of-life instruments exist.
 |
In retina, vitreoretinal interventions can improve a patient’s quality of life (QOL) from less than a percentage point for genetic testing to 20 percent or more for anti-VEGF treatments for neovascular age-related macular degeneration. The table on page 32 lists QOL measurements for a variety of vitreoretinal procedures.
QOL measurements can help retina specialists when evaluating treatments for patients. But what does a 20-percent improvement in QOL mean, exactly? In this article, we’ll explain what that means and look at existing tools for measuring quality after medical interventions, the limitations of those tools, and how they apply to outcomes in vitreoretinal interventions.
Comparing Apples, Oranges
The major outcomes of clinical trials are typically specific to a specialty. Unless the primary outcome is death, they are very difficult to compare. For example, the Early Treatment Diabetic Retinopathy Study (ETDRS) utilized a three-line improvement in vision (20/80 to 20/40) as a major outcome.2 The COMPASS Trial, which randomized patients with lower-extremity peripheral artery disease to aspirin, rivaroxaban (Xarelto, Janssen) or aspirin/rivaroxaban, used four major outcomes: number of hospitalizations; major adverse cardiovascular events; limb amputations; and death.3 Needless to say, it is impossible to compare the results of the ETDRS and COMPASS Trials utilizing the primary outcomes. So it is for thousands of clinical trials. (Incidentally, compared to aspirin alone, aspirin plus rivaroxaban decreased the incidence of major adverse limb events by 43 percent [p=0.01] and limb amputations by 58 percent [p=0.01].) 3
Despite the increasing popularity of quantifying QOL, most clinical trials have not incorporated it into their primary outcomes. The result is that patient opinions regarding a specific disease state are often disregarded. Furthermore, while the specialty-specific, 25-question National Eye Institute Visual Function Questionnaire QOL instrument (NEI-VFQ 25) provides considerable information in comparing ophthalmic clinical trials, it is difficult to compare such specialty-specific QOL instrument outcomes across different specialties.4
As payers and health systems place more emphasis on patient-centered care,5 soliciting QOL estimates that are comparable across specialties seems reasonable.6
A conservative estimate holds that more than 27 million different input variables can comprise a cost-utility, or cost-effectiveness, analysis. Changing even one variable can prevent the comparability of two or more cost-utility analyses.7 In regard to QOL instruments and respondents, thousands of different input variables exist, and they are different enough that they can prevent comparability of the QOL results.6
Generic QOL Instruments
Two variants of QOL instruments exist: generic and specialty-specific.6
Generic instruments can theoretically be utilized to quantify the QOL associated with a health state (one or more diseases) or intervention across all specialties. Included among these instruments are:
rating scales that ask patients to rate quality measures on a scale of zero to 100;
the Short Form 36 and 12 surveys (SF-36, SF-12) that pose 36 and 12 questions, respectively;
Quality of Well-Being (QWB) Scale;
the Karnofsky Performance Status Scale; and
different utility variants.6
Despite the hypothetical wide applicability of generic QOL instruments, they vary widely in terms of sensitivity to a health state, as well as reliability (reproducibility) and construct validity. Their utility to actually measure what they are intended to measure can be questionable.
 |
We prefer utility analyses, in particular the time trade-off variant, for evaluations of ophthalmological health states. Utility analyses employ patient preferences. Patients can typically give up something of value (time of life, lesser risk of immediate death, money, etc.) to hypothetically improve their health state, or give up nothing and remain in the same health state.6 The main variants include time trade-off utility analysis, standard gamble utility analysis, willing-to-pay utility analysis and multi-attribute utility analysis.6
• Time trade-off utility analysis. We prefer the time trade-off variant because it appears to have greater sensitivity, reliability and construct validity for ophthalmic health states than other variants.8–10 The anchors for utility analysis are usually 0.00 (death) and 1.00 (perfect health).
Visual time trade-off utilities have been shown to be typically unaffected by gender, age, ethnicity, level of education, wealth, comorbidities11 and country of origin (in developed countries).7 They correlate most closely with vision in the better-seeing eye and decease as the vision in the better eye decreases.12 Utilities more closely correlate with the degree of vision loss rather than the underlying cause of vision loss. 13
• Bias. Some authors have advocated the use of utility analysis in a manner that biases against the elderly and the disabled.14 The overall, mean systemic utility associated with each group is less than normal because of their associated disease profile. Let us assume the overall systemic utility is 0.80 in the average 85-year-old man.
Accordingly, if an ophthalmic or other intervention improves a patient’s quality of life to 0.95, the patient will only be assigned an 0.80 utility because that is his overall systemic utility. A younger person or otherwise healthy individual will be assigned the 0.95. In essence, the elderly and disabled accrue less benefit from an intervention than the average person who is younger and has an overall utility of 1.00. We strongly disagree with this form of discrimination and do not believe the public in the United States will accept it.
• Utility respondents. The respondent cohort—community, experts, physicians, researchers, administrators, surrogates—is as important as standardization of the instrument used to assess QOL. As an example, ophthalmologists, when asked to estimate the QOL associated with different levels of severity of AMD, underestimated the adverse effect of the disease by 96 to 750 percent compared to the actual patients with AMD.15 Medical students, non-ophthalmologists and the general community were even worse in estimating the adverse effects of vision loss upon patient QOL.16,17
All of the QOL values in this article were derived from ophthalmic patients using time trade-off utility analysis. Thus, all are comparable, unlike those from papers utilizing different QOL instruments and varied respondents.
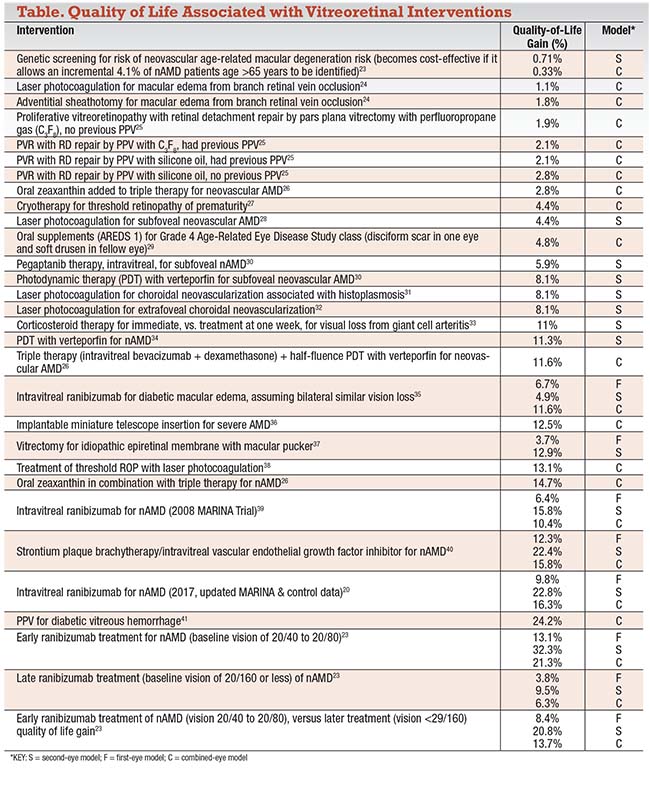 |
• One eye or two? Ophthalmic interventions are unique in that we have two eyes. Research has shown that the treatment of one eye, when the fellow eye still has good vision (first-eye model) confers less QOL gain than treatment of the second eye when vision in the first eye has already been lost to a disease (second-eye model).18 A combined-eye model, in which integrated first-eye and second-eye models are weighted, is preferable and most closely simulates the actual clinical condition. The table lists the types of vision utility models utilized.
Specialty-specific Instruments
More numerous than the generic instruments, hundreds of specialty-specific instruments exist. They include the NEI-VFQ 25 questionnaire), the Modified Rankin Scale for stroke, the American College of Rheumatology Classification of Global Functional Status in Rheumatoid Arthritis and the GOLD Classification (Global Initiative for Chronic Obstructive Lung Disease).6 These instruments can provide good comparability information within specialties or subspecialties, but typically have limited applicability for health states and interventions across specialties due to different sensitivities, reliability difficulties and questionable construct validity.6
Why QOL of Vitreoretinal Interventions Is Different
Many authors describe patient value gain (improvement in quality of life and/or length of life) in terms of QALY (quality-adjusted life-year) gain; most do not elect to calculate the percent QOL gain as we do in our Value-Based Medicine analyses. Because ophthalmic interventions do not usually affect length of life, patient value gain is most often the same as QOL gain and can be readily calculated as a percentage.
The QOL gains shown are all associated with the Center for Value-Based Medicine; thus, all are comparable. A number of factors can prevent comparability among QOL analyses. They include different utilities, unlike utility respondents, lack of a 3-percent annual discount rate as recommended by the Panel on Cost-Effectiveness in Health and Medicine,19 utility calculations variations, and lack of sufficient data in the manuscript. The QOL gains listed in the table were all calculated as part of a cost-utility analysis. The cost-utility ratios are not listed and are outside the scope of this article.
A note about the 3-percent discount rate: This applies to both QOL (patient value gain) and dollar value, which is discounted due to the time value of money (money in the future is worth less than money today); and the fact that good health now is worth more than it is in the future because good health now can enable a person to earn money that can be invested.
An additional note on the ophthalmic models, though not the systemic models, is warranted. Typically, first-eye models (F) yield the lowest QOL gain because the fellow eye is presumed to be normal. If the fellow eye has 20/20 vision and the vision in the treated eye improves from 20/100 to 20/60, most patients do not notice an appreciable difference in their overall QOL. However, when vision in the second eye deteriorates, and the first eye already has vision loss, there is typically a much larger QOL gain from the same intervention vs. when it was performed in the first-eye model.6
Combined-eye Model
The combined-eye model weights to the first-eye and second-eye models to provide the best simulation of the clinical scenario. The first-eye model alone tends to underestimate QOL gain, while the second-eye model tends to overestimate it.6 For example, in a follow-up MARINA trial cost-utility analysis in 2017 assessing ranibizumab (Lucentis, Roche/Genentech) for the treatment of nAMD,20 the first-eye model QOL gain of 16.3 percent underestimated the combined-eye model by 41 percent. The second-eye model overestimated the QOL gain by 40 percent.
Of special note is the dramatic gain in QOL in nAMD with ranibizumab for early treatment (baseline vision 20/40 to 20/80) vs. late treatment (vision < 20/160) (Table). Taking both eyes into account, early treatment results in a 21.3 percent QOL gain vs. a 6.3 percent QOL gain for late treatment.
Overall, there is a great range of QOL gains associated with vitreoretinal interventions, most of which compare favorably with nonvitreoretinal ophthalmic and systemic interventions. We did not address the associated costs of the interventions listed, but many have a considerable financial return-on-investment to society for the direct medical costs expended.
Economist William Nordhaus, PhD, reported that health-care advances accounted for 50 percent of the wealth accrued in the United States in the 20th century.22 We should be pleased that our interventions provide not only improvements in QOL, but also make the United States a wealthier country by giving patients greater independence and the ability to work. RS
REFERENCES
1. Salek S. The Compendium of Quality of Life Instruments. Pasadena, Calif.; John Wiley and Sons Inc.;1999.
2. Early Treatment Diabetic Retinopathy Study Research Group. Photocoagulation for diabetic macular edema. Early Treatment Diabetic Retinopathy Study report number 1. Arch Ophthalmol. 1985;103:1796-1806.
3. Anand SS, Caron F, Eikelboom JW, Bosch J, et al. for the COMPASS Trial Investigators. Major adverse limb events and mortality in patients with peripheral artery disease: COMPASS Trial. J Am Coll Cardiol. 2018;71:2306-2316.
4. Kay S, Ferreira A. Mapping the 25-item National Eye Institute Visual Functioning Questionnaire (NEI VFQ-25) to EQ-5D utility scores. Ophthalmic Epidemiol. 2014;21:66-78.
5. Morris MS, Hawn MT. Beyond readmission: improving patient-centered care. J Surg Res. 2018; pii: S0022-4804(18)30094-5.
6. Brown MM, Brown GC, Sharma S. Evidence-based to Value-based Medicine. Chicago, Ill.; American Medical Association Press; 2005.
7. Brown GC, Brown MM, Kertes P. Comparative effectiveness and cost-effectiveness analyses: 27,000,000 possible input variants. Evidence-Based Ophthalmol. 2011;12:52-7.
8. Brown GC, Brown MM, Sharma S, Beauchamp G, Hollands H. The reproducibility of ophthalmic utility values. Trans Am Ophthalmol Soc. 2001;99:199-203.
9. Hollands H, Lam M, Pater J, Albiani D, Brown GC, Brown MM, Cruess AF, Sharma S. Reliability of the time trade-off technique of utility assessment in patients with retinal disease. Can J Ophthalmol. 2001;36:202-209.
10. Sharma S, Brown GC, Brown MM, Hollands H, Robbins R, Shah G. Validity of the time trade-off and standard gamble methods of utility assessment in retinal patients. Br J Ophthalmol. 2002;86:493-496.
11. Real FJ, Brown GC, Brown HC, Brown MM. The effect of comorbidities upon ocular and systemic health-related quality of life. Br J Ophthalmol. 2008;92:770-774.
12. Brown MM, Brown GC, Sharma S, Landy J. Health care economic analyses and value-based medicine. Surv Ophthalmol. 2003;48:204-223.
13. Brown MM, Brown GC, Sharma S, Landy J. Quality of life with visual acuity loss from diabetic retinopathy and age-related macular degeneration. Arch Ophthalmol. 2002;120:481-484.
14. Feeny D, Krahn M, Prosser LA, Salomon JA. Valuing health outcomes. In: Neumann PJ, Sanders GD, Russell LB, Siegel JE, Ganiats TG (eds). Cost-Effectiveness in Health and Medicine, Second edition. New York, N.Y.; Oxford University Press; 2017:167-199.
15. Brown GC, Brown MM, Sharma S. Difference between ophthalmologist and patient perceptions of quality-of-life associated with age-related macular degeneration. Can J Ophthalmol. 2000;35:27-32.
16. Chaudry I, Brown GC, Brown MM. Medical student perceptions of quality-of-life associated with vision loss. Can J Ophthalmol. 2015;50:217-24.
17. Stein JD, Brown MM, Brown GC, Sharma S, Hollands H. Quality of life with macular degeneration. Perceptions of patients, clinicians and community members. Br J Ophthalmol. 2003;87:8-12.
18. Brown MM, Brown GC, Sharma S, Brown H, Busbee B. Quality-of-life associated with unilateral and bilateral good vision. Ophthalmology 2001;108:643-647.
19. Siegel JE, Weinstein MC, Russell LB, Gold MR. Panel on Cost-Effectiveness in Health and Medicine: Recommendations for reporting cost-effectiveness analysIs. JAMA. 1996; 276:1339-1341.
20. Brown GC, Brown MM, Lieske HB, Brown KS, Colman S, Tran I. A Value-Based Medicine analysis of ranibizumab for neovascular macular degeneration. The return-on-investment and wealth of the nation. Int J Retina Vit. 2017) 3:5 DOI 10.1186/s40942-016-0058-3.
21. Boyer DS, Antoszyk AN, Awh CC, for the MARINA Study Group, et al. Subgroup analysis of the MARINA study of ranibizumab in neovascular age-related macular degeneration. Ophthalmology. 2007;114:246–52.
22. Nordhaus WD. The health of nations: The contribution of improved health to living standards. Working paper 881. Cambridge, Mass.; National Bureau of Economic Research; 2002: 37-38.
23. Brown GC, Brown MM, Lieske HB, Lieske PA, Brown KS. A Value-Based Medicine analysis of genetic testing for neovascular macular degeneration. Int J Ret Vitreous. 2015:1:19
24. Brown GC, Brown MM, Sharma S, Busbee B, Brown H. Incremental cost-effectiveness of laser therapy for visual loss secondary to branch retinal vein occlusion. Ophthalmic Epidemiology. 2002;9:1-10.
25. Brown GC, Brown MM, Sharma S, Busbee B. A cost-utility analysis of interventions for proliferative vitreoretinopathy. Am J Ophthalmol. 2002;133:365-372.
26. Olk RJ, Peralta E, Gierhart DL, Brown MM, Brown GC. Triple combination therapy and zeaxanthin for the treatment of neovascular age-related macular degeneration: An interventional comparative study and cost-effective analysis. Int J Retina Vitreous. 2015;9:22.
27. Brown GC, Brown MM, Sharma S, Tasman W, Brown H. Cost-effectiveness of therapy for threshold retinopathy of prematurity. Pediatrics. 1999;104:e47.
28. Brown GC, Brown MM, Sharma S. Incremental cost-effectiveness of laser photocoagulation for subfoveal choroidal neovascularization. Ophthalmology 2000;107:1374-1380.
29. Sharma S, Bakal J. Center for Value-Based Medicine internal files.
30. Brown GC, Brown MM, Brown HC, Kindermann S, Sharma S. A value-based medicine c:157-60.comparison of interventions for subfoveal neovascular macular degeneration. Ophthalmology. 2007;114:1170-1178.
31. Brown GC, Brown MM, Sharma S, Busbee B, Brown H. Incremental cost-effectiveness of laser photocoagulation for choroidal neovascularization associated with histoplasmosis. Retina. 2000;20:331-337.
32. Busbee B, Brown MM, Brown GC, Sharma S. A cost-utility analysis of laser photocoagulation for extrafoveal choroidal neovascularization. Retina 2003;23:279-287.
33. Bakal J, Sharma S. The value component of evidence-based medicine: The utility analysis of early versus late corticosteroid treatment for giant cell arteritis. Evidence-based Ophthalmol. 2006;7:157-160.
34. Sharma S, Brown GC, Brown MM, Hollands H, Shah GK, Sharma SM. Improvement in quality of life from photodynamic therapy: a Canadian perspective. Can J Ophthalmol. 2001;36:332-338.
35. Brown GC, Brown MM, Turpcu A, Rajput Y. The cost-effectiveness of ranibizumab therapy for the treatment of diabetic retinopathy. Ophthalmology. 2015;122:1416-1425.
36. Brown GC, Brown MM, Lieske HB, Lieske PA, Brown KS. Comparative effectiveness and cost-effectiveness analyses on the Implantable miniature telescope. Ophthalmology. 2011;118:1834-1143.
37. Gupta O, Brown GC, Brown MM. A value-based medicine cost-utility analysis of idiopathic epiretinal membrane surgery. Am J Ophthalmol. 2008;145:923-928.
38. Brown GC, Brown MM, Brown H, Peet JS. A value-based medicine analysis of ranibizumab (MARINA Study) for the treatment of subfoveal neovascular macular degeneration. Ophthalmology. 2008;115:1039-1045.
39. Brown MM, Brown GC, Brown HC, Irwin B, Roth Z. Comparative effectiveness and cost-effectiveness analyses of VEGF-A inhibitor and Sr 90 brachytherapy for neovascular macular degeneration. Evidence-Based Ophthalmol. 2009;10:107-122.
40. Brown GC, Brown MM, Lieske HB, Lieske PA. Preference-based cost-effectiveness: a review and relevance of value-based medicine for vitreoretinal interventions. Curr Opin Ophthamol. 2012;23:163-174.



