Recently reported results of the Phase Ib trial of the investigational anti-VEGF agent KSI-301 (Kodiak Sciences) revealed promising data on the safety, efficacy and durability of the drug in patients with previously untreated exudative retinal diseases. Charles C. Wykoff, MD, PhD, reported the first results from the trial last month at Retina 2019 Subspecialty Day at the American Academy of Ophthalmology.1
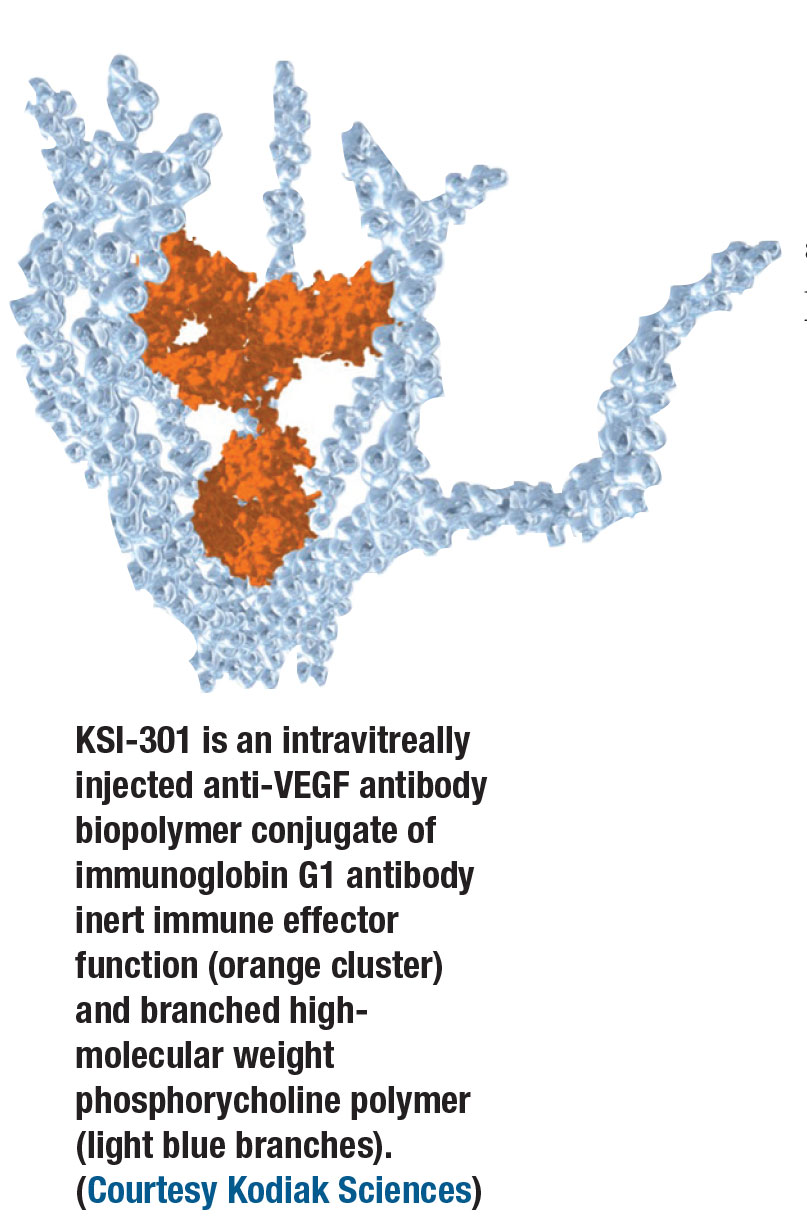
KSI-301 is an intravitreal agent based on a novel platform, called antibody biopolymer conjugate, or ABC, that uses a large molecular structure to bind to and inhibit vascular endothelial growth factor.
In a press release reporting the latest Phase Ib results, Kodiak Sciences chief medical officer Victor Perlroth, MD, said the Phase Ib results support the company’s intent to develop KSI-301 to reduce the treatment burden of existing anti-VEGF treatments while improving visual acuity. The findings have enabled Kodiak Sciences to begin enrolling patients in a global, multicenter, randomized Phase II trial called DAZZLE. This trial will eventually enroll at least 368 patients and compare KSI-301 on an individualized dosing regimen of three to five months and aflibercept (Eylea, Regeneron Pharmaceuticals) every eight weeks.
Here, Dr. Wykoff answers questions about the Phase Ib and Phase II trials. Dr. Wykoff, who is also chief clinical editor of Retina Specialist Magazine, is a paid consultant and researcher for Kodiak Sciences.
Q. What makes KSI-301 different from other anti-VEGF agents that are available?
Mechanistically, KSI-301 binds to VEGF-A as the other primary anti-VEGF agents. What makes KSI-301 unique is the projected durability of this effect inside of the eye. The antibody biopolymer conjugate platform on which KS-301 is based has been engineered specifically for increased durability. It has two components, a specific anti-VEGF IgG1 antibody with an inert immune effector function that is covalently and stably linked to an intentionally high molecular weight, optically clear phosphorycholine biopolymer.
The concept is to maximize intraocular durability by leveraging size and molar dose. The molecular weight of KSI-301 is 950 kilodaltons vs. 48 kDa for ranibizumab
(Lucentis, Roche/Genentech) and 115 kDa for aflibercept. This, combined with a 3.5-fold greater molar dose than aflibercept, leads to an estimated intraocular anti-VEGF effect at three months that has been calculated, based on preclinical studies, to be 1,000-fold greater than aflibercept.
 |
Q. What did the preclinical studies reveal about the potential efficacy of KSI-301?
Preclinical studies have demonstrated three key effects. First, in rabbit models the drug appeared to have a substantial durability benefit compared to previous generation anti-VEGF agents, with an estimated half-life in the rabbit retina to choroid of approximately 10 to 15 days. Second, it demonstrated excellent bioavailability at the target tissues—the retina and choroid. Third, because of its inert Fc domain, when the molecule does diffuse from the eye into systemic circulation, the anticipated primary route of exit from the eye, it clears from systemic circulation rapidly with a systemic half-life of less than one day, much less than bevacizumab (Avastin, Roche/Genentech, 11.5 days).
Q. What were the key findings of the Phase Ia trial previously reported.
That Phase Ia trial involved nine patients with diabetic macular edema. They received a single dose of KSI-301, which showed a durability of effect through 12 weeks without any drug-related adverse events.
Q. What was the design of the Phase Ib trial?
The Phase Ib trial involved 105 patients—35 each with wet age-related macular degeneration, DME and retinal venous occlusive disease. These patients were randomized to 2.5- or 5-mg doses of KSI-301. All patients were given three monthly loading doses and then evaluated monthly, receiving retreatment when prespecified anatomic and/or visual criteria were met.
Q. What are the key findings of the Phase Ib trial that you reported at the AAO?
This is an ongoing study and the data I presented on behalf of my co-investigators were interim results. In the nAMD group, at 16 weeks, best-corrected visual acuity has improved 5.4 letters on average, and central subfield thickness decreased 72 µm on average. Eighty percent of these patients were able to be extended four months or longer before needing their first retreatment after the three loading doses.
 |
Among the DME population, BCVA improved an average of 8.4 lines at 16 weeks and CST improved 140 µm on average. Again a majority, or 82 percent, have so far been able to be extended longer than three months after the last loading dose, and some have been extended to six months before meeting retreatment criteria. Furthermore, all DME patients had either improved or maintained their diabetic retinopathy severity level and none developed a proliferative DR event.
In RVO, BCVA improved 21.3 letters on average at 16 weeks and CST decreased 353 µm (the baseline average CST was approximately 250 µm greater in this group than in the nAMD and DME groups). Among the RVO patients, 56 percent have so far been able to be extended beyond three months after their last loading dose.
Equally important to efficacy and durability, the safety profile of KSI-301 appears excellent, with no intraocular inflammatory events and no drug-related adverse events in 316 total treatments across the Phase I program thus far.
Q. What are the next steps for KSI-301?
The pivotal Phase II DAZZLE study is enrolling treatment-naïve nAMD patients and randomizing them to either 5-mg KSI-301 or aflibercept. KSI-301 is being dosed every 12, 16 or 20-weeks depending on prespecified disease activity assessments and the primary endpoint is at one year.
Q. Where would KSI-301 potentially fit in the retina specialist’s toolbox for treating exudative retinal disease?
In real-world wet AMD dosing, multiple reports have documented that patients are not receiving the optimal number of injections based on current standard-of-care agents. The hope is that by developing more durable treatments, we are going to improve real-world outcomes.
Decreasing treatment burden is good, but at the same time, because we aren’t dosing frequently enough in the real world, patients aren’t achieving their long-term, maximum potential visual outcomes. More durable agents will deliver a meaningful advantage if they can address this real-world challenge. RS
REFERENCE
1. Wykoff CC. Extended durability in exudative retinal diseases using the novel intravitreal anti-VEGF antibody biopolymer conjugate KSI-301: Results from the Phase 1b study in patients with AMD, DME and RVO. Presented at American Academy of Ophthalmology Subspecialty Day Retina 2019; October 11, 2019; San Francisco, CA..



