 |
Here, we explore emerging treatment modalities for wet age-related macular degeneration that aim to overcome the shortcomings of existing anti-VEGF agents by providing sustained improvement of vision while reducing the frequency of injections and clinic visits.
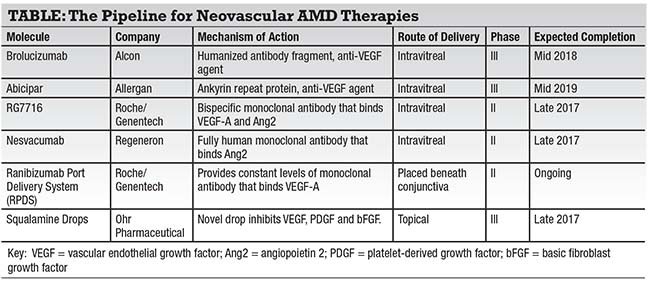
Brolucizumab
Formerly known as RTH258 and ESBA1008, brolucizumab (Novartis) is a humanized antibody fragment with a molecular weight (26 kDa) much smaller than that of any currently available anti-VEGF agent.2 Investigators have proposed that the smaller molecular weight of brolucizumab may result in greater ocular tissue penetration and higher concentrations of the drug being localized in the retina.
Animal studies have elucidated these benefits, having shown a fourfold lower systemic exposure compared to ranibizumab (Lucentis, Genentech), a tolerability to higher doses and a high affinity for vascular endothelial growth factor.3 Early human studies in patients who have been diagnosed with neovascular AMD have shown promising
 |
potent and longer lasting than a single dose of ranibizumab.4 Phase II studies comparing brolucizumab to aflibercept (Eylea, Regeneron) have shown noninferiority, providing the basis for the ongoing phase III studies, HAWK and HARRIER. These studies are fully enrolled with an estimated completion time of mid-2018.5
Abicipar
Abicipar (Allergan), formerly AGN-150998, is a designed ankyrin repeat protein (DARPin) that acts as an anti-VEGF agent. Abicipar has small-molecular weight structure with the added benefit of having a polyethylene tail designed to provide the drug with a longer intravitreal half-life.6 During the Phase II REACH study, 25 patients were randomized to abicipar 1 mg, 23 patients to abicipar 2 mg, and 16 patients to ranibizumab 0.5 mg.
Results from this study showed an improvement of mean visual acuity from baseline of 9 letters for abicipar 2 mg and 7.1 letters for abicipar 1 mg compared to 4.7 letters for ranibizumab 0.5 mg.
Although this study was not powered to show statistical significance, it provided promising results that paved the way for a Phase III program consisting of two trials: CEDAR and SEQUOIA.6 These randomized, double-masked studies comparing abicipar to ranibizumab have each recruited 900 patients and are fully enrolled. The estimated completion date is mid-2019.7
RG7716
RG7716 (Genentech) is a bispecific monoclonal antibody that uses CrossMab technology to bind VEGF-A on one arm and angiopoietin 2 (Ang2) on the other arm.
Ang2 plays a role in suppressing phosphorylation of Tie2 (Tyrosine kinase with immunoglobulin-like and epidermal growth factor like domains 2) receptor.
Tie2 receptor is phosphorylated in the active state and has been implicated in the modulation of vascular permeability. Upregulation of Ang2 has been observed in proangiogenic conditions such as diabetic retinopathy, diabetic macular edema, AMD and retinal vein occlusion leading to decreased Tie2 activation and subsequent vascular leakage and neovascularization.8-10
A Phase I study in patients with difficult-to-treat neovascular AMD deemed RG7716 “safe and well-tolerated” among those with no observed dose-limiting toxicities or unexpected adverse events.11
During the Phase I study, patients were divided into two treatment groups: a single ascending-dose group that received 0.5-, 1.5-, 3- and 6-mg doses of RG7716; and a multiple ascending-dose group that received three monthly treatments each with 3- and 6-mg doses.
 |
The single ascending-dose group documented a median improvement in visual acuity of 7 letters, while the multiple ascending group had a median improvement of 7.5 letters.11
A randomized, double-masked Phase II study, AVENUE, is looking at efficacy of RG7716 vs. ranibizumab in the treatment of choroidal neovascularization secondary to AMD. AVENUE is fully enrolled and has an estimated completion by the end of the year.12
Another randomized Phase II trial looking at extended dosing interval with RG7716, STAIRWAY, is also fully enrolled and is expected to finish in first quarter of 2017.13
Nesvacumab/ Aflibercept
Nesvacumab (Regeneron) is a fully human monoclonal antibody that inactivates Ang2 with high affinity. In combination with an anti-VEGF agent, such as aflibercept (Eylea, Regeneron), nesvacumab has the potential to prevent the pathological process of angiogenesis in neovascular AMD.
The Phase I study evaluating the safety and tolerability of nesvacumab/aflibercept in 20 patients with neovascular AMD or DME reported visual and anatomical improvements at all dose levels.14 This trial reported no dose-limiting toxicities, ocular inflammation or unexpected systemic effects.
These results have led to a Phase II study (ONYX), now fully enrolled, that will assess the efficacy of intravitreal nesvacumab/aflibercept compared to aflibercept in patients with neovascular AMD.15 Estimated completion date for ONYX is by the end of the year.
Ranibizumab Port Delivery
The ranibizumab port delivery system (RPDS, Genentech) is a port placed beneath the conjunctiva that physicians can refill with a custom refill needle.16 The device is designed to release ranibizumab over a period of months, potentially reducing the burden of frequent injections.
An implantable delivery system like this has the potential to provide patients with a constant level of treatment and ultimately become a tool to better manage neovascular AMD and improve long-term visual outcomes.
The Phase I study showed RPDS to be well-tolerated with documented improvement in best-corrected visual acuity compared to monthly injections.17 Genentech has received fast-track designation from the Food and Drug Administration and has initiated a randomized, double blind, Phase II study (LADDER) that is currently under way.18 LADDER will assess the efficacy and safety of the delivery system.
Squalamine Drops
Squalamine (OHR Pharmaceutical) is a topical anti-angiogenic drop with a novel intracellular mechanism of action. The drop works by inhibiting multiple growth factors, including VEGF, platelet-derived growth factor (PDGF) and basic fibroblast growth factor (bFGF).
The topical formulation of Squalamine, called OHR-102, is currently being evaluated in Phase III trial. The MAKO study, is a multicenter, randomized, double masked, placebo controlled clinical trial looking at monthly ranibizumab and either Squalamine drops or placebo drops twice daily.19 The study is fully enrolled and data is expected in early 2018. RS
REFERENCES
1. Holz FG, Tadayoni R, Beatty S, et al. Key drivers of visual acuity gains in neovascular age-related macular degeneration in real life: findings from the AURA study. Br J Ophthalmol. 2016 Mar 30.
2. Positive phase II data highlights benefits of Alcon’s RTH258 for patients with neovascular (wet) age-related macular degeneration [press release]. Basel, Switzerland: Novartis; February 27, 2017. https://www.novartis.com/news/media-releases/positive-phase-ii-data-highlights-benefits-alcons-rth258-patients-neovascular. Accessed May 15, 2017.
3. Dugel P. Results of ESBA 1008, a single-chain antibody fragment for the treatment of neovascular AMD. Paper presented at: Annual meeting of the American Society of Retina Specialists; San Diego, CA; August 9-13, 2014.
4. Barakat MR, Dugel PU. New developments for the treatment of exudative and nonexudative AMD. Retinal Physician. 2015:12(10):26-36.
5. Singerman LJ. Brolucizumab vs. aflibercept treatment for neovascular AMD. Paper presented at: Angiogenesis, Exudation, and Degeneration 2017; Feb. 11, 2017; Miami.
6. Bethke W. Meet your next AMD drug. 2015;22(8):38-42.
7. Safety and efficacy of abicipar pegol in patients with neovascular age-related macular degeneration. ClinicalTrials.gov. https://clinicaltrials.gov/ct2/show/NCT02462486. Accessed March 09, 2017.
8. Huang J, Bae JO, Tsai JP, et al. Angiopoietin-1/Tie-2 activation contributes to vascular survival and tumor growth during VEGF blockade. Int J Oncol. 2009;34;79–87.
9. Khanani AM. The Tie2 activator AKB-9778, used In combination with ranibizumab, enhances reduction of diabetic macular edema compared to ranibizumab monotherapy. Invest Ophthalmol Vis Sci. 2016;57(12);no pagination specified. http://iovs.arvojournals.org/article.aspx?articleid=2560785 Accessed May 15, 2017.
10. Campochiaro PA, Khanani AM. Enhanced benefit in diabetic macular edema from AKB-9778 Tie2 activation combined with vascular endothelial growth factor suppression. Ophthalmology. 2016:123:1722-1730.
11. Dugel P. A novel anti-VEGF/anti-angiopoietin2 bispecific monoclonal antibody for wet age-related macular degeneration and diabetic macular edema. Presented at: American Society of Retina Specialists annual meeting; August 9-14, 2016; San Francisco, CA.
12. AVENUE: A proof-of-concept study of RG7716 in participants with choroidal neovascularization (CNV) secondary to age-related macular degeneration (AMD). ClinicalTrials.gov. https://clinicaltrials.gov/ct2/show/record/NCT02484690. Accessed March 09, 2017.
13. Study to evaluate RO6867461 (RG7716) for extended curability in the treatment of neovascular age-related macular degeneration (nAMD) (STAIRWAY). ClinicalTrials.gov. https://clinicaltrials.gov/ct2/show/NCT03038880. Accessed May 15, 2017.
14. Brown DM. Phase 1 study of combination therapy with nesvacumab and aflibercept for neovascular AMD and diabetic macular edema. Presented at: American Academy of Ophthalmology annual meeting; Oct. 14-18, 2016; Chicago, IL.
15. Anti-angiopoeitin 2 plus anti-vascular endothelial growth factor as a therapy for neovascular age related macular degeneration: Evaluation of a fixed combination intravitreal injection (ONYX). ClinicalTrials.gov. https://clinicaltrials.gov/ct2/show/record/NCT02713204. Accessed March 09, 2017.
16. Genentech announces first milestone payment to device-maker ForSight VISION4, Inc. in development of sustained delivery Lucentis [press release]. San Francisco, CA: Genentech; January 13, 2012. https://www.gene.com/media/press-releases/13808/2012-01-13/genentech-announces-first-milestone-paym. Accessed May 15, 2017.
17. Helzner J. Sustained-release delivery of Lucentis studied. Retinal Physician. 2016;13(1):10-12.
18. Study of the efficacy and safety of the ranibizumab port delivery system for sustained delivery of ranibizumab in participants with subfoveal neovascular age-related macular degeneration (LADDER). ClinicalTrials.gov. https://clinicaltrials.gov/ct2/show/NCT02510794?term=LADDER&rank=7. Accessed May 15, 2017.
19. Ohr Pharmaceutical provides update on ongoing squalamine clinical trial in wet-AMD [press release]. New York, NY: Ohr Pharmaceutical; April 10, 2017. http://www.ohrpharmaceutical.com/media-center/press-releases/detail/482/ohr-pharmaceutical-provides-update-on-ongoing-squalamine. Accessed May 15, 2017.



