 |
 |
 |
Retinoblastoma continues to be the most common primary intraocular malignancy of childhood. However, enucleation continues to be the international gold standard of management. During the last 20 years, globe-salvaging therapies with chemoreduction and focal consolidation have significantly improved the morbidity associated with retinoblastoma RB therapy.
Still, some developing countries with limited access to medical care continue to struggle with the high morbidity and mortality associated to the natural course of the disease.1,2 Today, novel treatments for advanced disease are actively being investigated to minimize the need of enucleation and limit the toxicities associated to therapy. This article reviews the most current clinical pearls in the management of retinoblastoma.
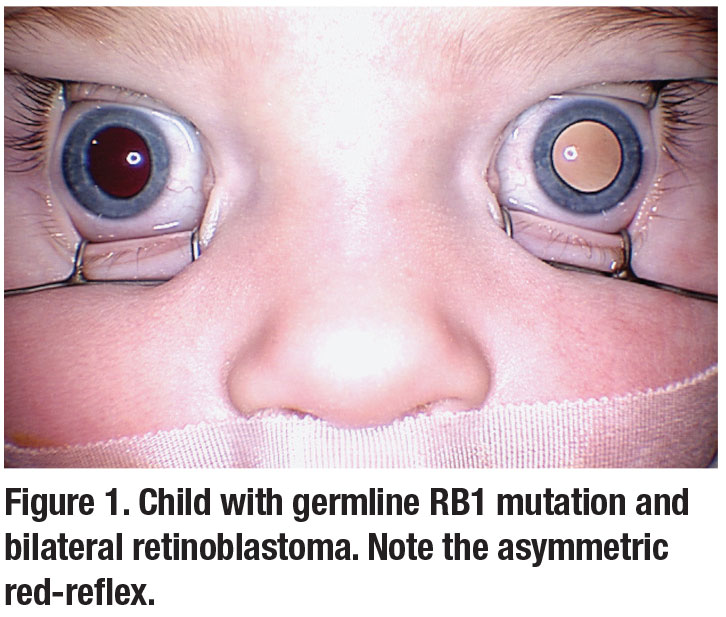
Retinoblastoma (RB) affects one in 15,000 to 20,000 live births.1 Early diagnosis is of critical importance because small tumors have the best prognosis. Leukocoria is the most common sign at initial presentation (Figure 1).1 However, small or peripheral tumors may not alter the red-reflex. Sensory strabismus is the second most common sign.2
Focus on local treatments
Therapies for RB have dramatically advanced during the last 10 years. A significant trend toward targeted primary therapy with selective intra-arterial and intravitreal chemotherapy is currently under way. Technological changes and strategies focus on local treatments due to decreased morbidity to patients and excellent tumor response. New treatments are providing new hope to patients, especially to those with the most severe disease.
 |
Management of RB tumors requires a multidisciplinary approach that may include an ocular oncologist, pediatric oncologist, pediatric ophthalmologist, pediatrician, interventional radiologist and ocular pathologist. Individualized treatment, considering factors such as the International Classification (IC) of RB, laterality, location of tumors, age of patient, family history and prior treatment must be considered,1,3 although RB treatment is aimed at child survival. Globe salvage and preservation of vision are important secondary goals. Early diagnosis remains the most crucial step in decreasing morbidity and mortality.2
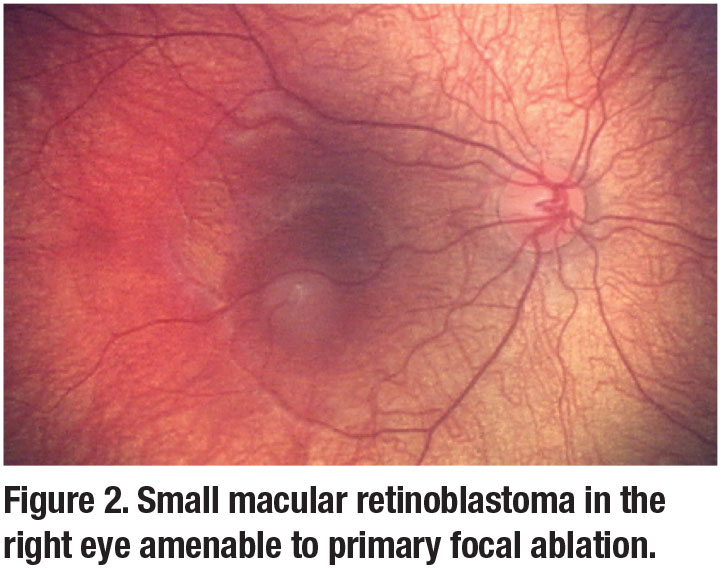
Treatment of small tumors (Figure 2) may only require transpupillary thermotherapy.4 Laser treatments may be repeated monthly until complete tumor regression is documented.5 Close follow-up with patients is important to monitor for recurrence. If recurrence is detected, systemic or intra-arterial chemotherapy may be considered.
Chemotherapy and chemoreduction
 |
The classic three-drug systemic treatment (carboplatin, vincristine and etoposide) has been associated with significant morbidity, and it routinely requires multiple cycles. Bone marrow suppression, ototoxicity, nephrotoxicity and risk of induction of secondary cancers have been reported.6-7 Controversy continues to surround the role of systemic chemotherapy in the prevention of trilateral RB.8-9 The combination of systemic and/or intra-arterial chemotherapy with focal ablative treatments has been shown to have better globe salvage rates than chemotherapy alone in both early and advanced RB.10-13
A recent study on macular retinoblastoma outcomes showed that chemoreduction with transpupillary thermotherapy of both foveal and extrafoveal tumors achieve control in 83 percent of R-E group V tumors.14 All tumors less than R-E group V achieved 100-percent control. Despite ablative foveal laser treatment, 56 percent of eyes had better than 20/80 visual acuity.
 |
Enucleation remains the standard treatment of group E RB tumors.15 Histopathologic analysis may determine if adjuvant treatment is necessary depending on high-risk criteria at the time of enucleation.16 Adjuvant therapy postenucleation has been shown to decrease metastasis in advanced RB from 24 to 4 percent of children.17
Intra-arterial chemotherapy
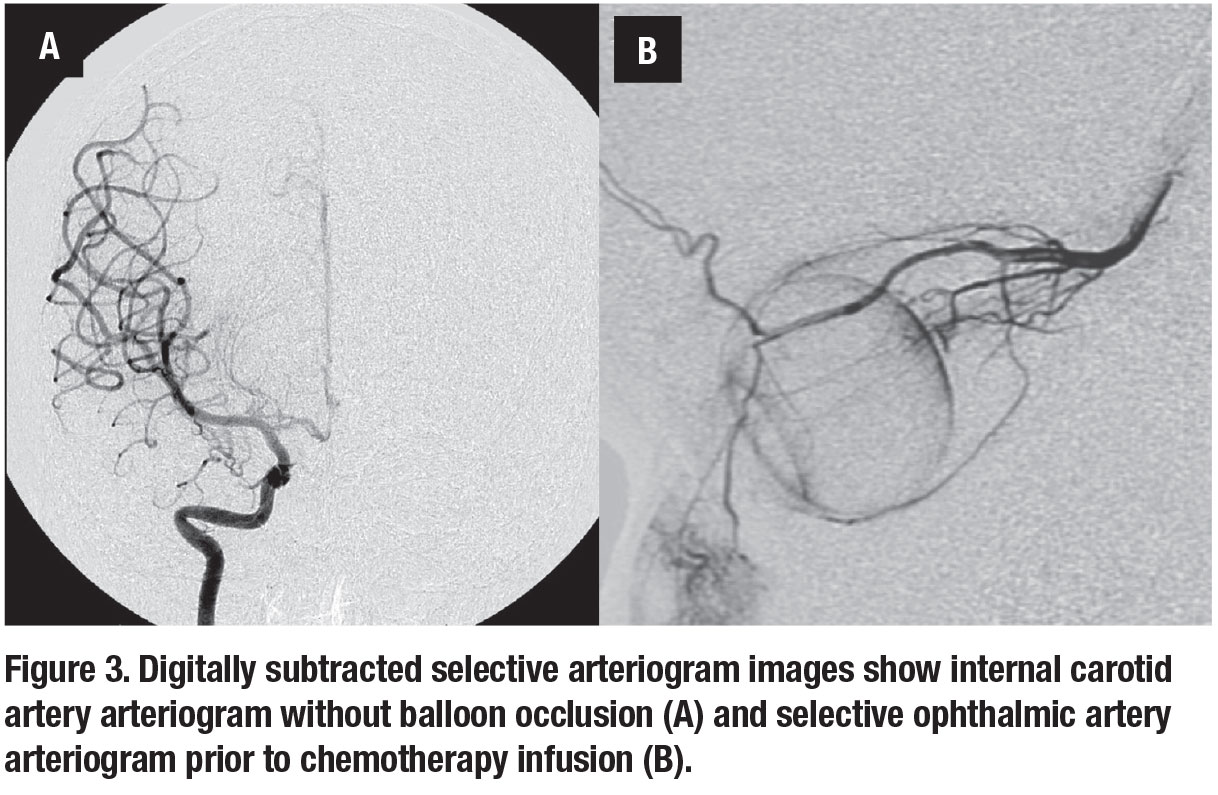
Physicians in Japan revolutionized the treatment of RB by infusing melphalan directly into the ophthalmic artery.18 The initial technique consisted of catheterization of the internal carotid artery and occlusion of a micro-balloon distal to the ophthalmic artery. During the temporary occlusion, melphalan was infused into the ophthalmic artery. The study involved 563 intra-arterial chemotherapy procedures in 187 patients with no reported serious complications, including stroke. The most common complications were mild transient bradycardia, periorbital erythema and swelling.18
Following the initial publication, many large centers had significant interest in developing the technique in patients with advanced RB. Subsequently, David Abramson, MD, and associates at Memorial Sloan-Kettering Cancer Center developed a technique that allowed treatment without the need to occlude the distal cerebral blood flow at the time of infusion (Figure 3).19
 |
Recent studies have validated the efficacy of intra-arterial chemotherapy.20-22 Interest in intra-arterial delivery of other chemotherapeutic agents has prompted various small studies. This strategy has been investigated to avoid melphalan dose restriction during bilateral therapy. Jasmine Francis, MD, and colleagues at Memorial Sloan-Kettering reported in 2012 that the use of single-agent carboplatin at doses ranging from 25 to 40 mg, and cumulative doses from 25 to 100 mg, in three cases where high-dose melphalan was needed in the contralateral eye and systemic toxicity limited the use of melphalan to one eye.23 They reported tumor regression with as little as one cycle and no systemic adverse effects.
Similar results have been reported with intra-arterial infusion of both carboplatin and topotecan.24 In addition, analysis of electroretinogram (ERG) responses following infusions containing carboplatin only and carboplatin with topotecan revealed no statistically significant change.23-24
 |
Three-drug intra-arterial treatment
Most recently, a trend toward three-drug intra-arterial treatment (carboplatin, melphalan and topotecan) has been reported.25 Twenty-six eyes of 25 patients received the three-drug chemotherapy for treatment of advanced retinoblastoma. Dose ranges were 2.5 to 7.5 mg of melphalan, 0.3 to 0.6 mg of topotecan and 25 to 50 mg of carboplatin. Median infusions per eye were two (range: two to four). The Kaplan-Meier estimate of ocular survival at 24 months was 75 percent. ERG showed improvement greater than 25 µV in four eyes (15 percent), loss greater than 25 µV in 12 eyes (46 percent) and no change greater than 25 µV in 10 eyes (39 percent).
Other large studies have also reported successful treatment with this regimen.21 These findings suggest that selective intra-arterial combination therapy with carboplatin and melphalan is effective in the treatment of RB and decreases the toxic window during treatment especially in patients that need bilateral therapy.
Sequential intravenous chemotherapy followed by intra-arterial chemotherapy (bridge chemotherapy) for young infants with retinoblastoma may be considered when cannulation of the ophthalmic artery isn’t possible.26 Further studies will elucidate the optimal timing for bridging.
Intravitreal chemotherapy
The significant tumoricidal effects reported with intra-arterial melphalan generated enthusiasm to study intravitreal delivery for vitreous seeding. However, the potential for tumor dissemination through the needle tract following intravitreal penetration has limited its use.
In 2012 researchers at Jules-Gonin Eye Hospital in Switzerland reported the first clinically documented case series of patients with retinoblastoma treated with intravitreal melphalan.27 The study included 122 intravitreal injections of melphalan in 23 eyes that had significant active vitreous seeding after primary therapy. Globe retention was achieved in 87 percent (20 of 23) of patients. Despite the confounding effects of concomitant chemotherapy, the study showed that intravitreal melphalan achieved unprecedented control of vitreous seeding.27
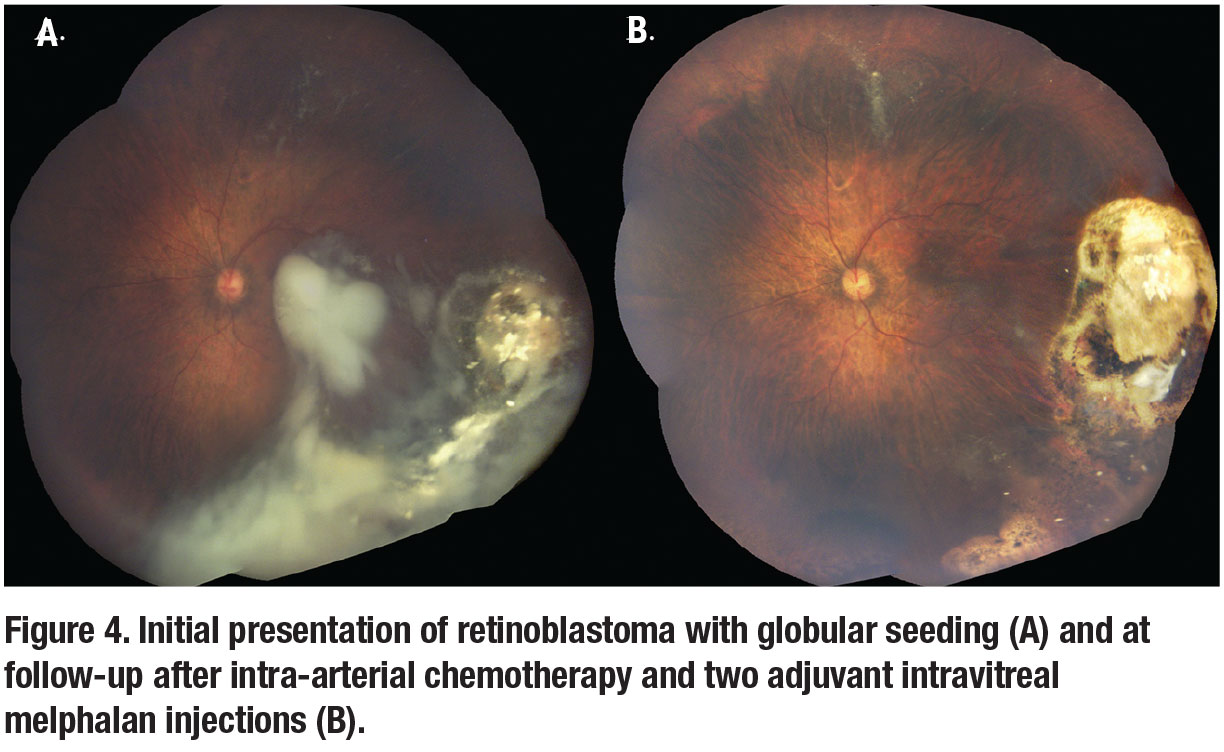 |
A recent bi-institutional cohort study evaluated the vitreous seed response after 475 intravitreal melphalan injections.28 The study included 87 eyes treated weekly (median dose, 30 µg) with a median of five treatments per eye (range, one to 12 times). The two-year Kaplan-Meier estimates for ocular survival and patient survival were 90.4 percent and 100 percent, respectively. Other authors have also reported on the efficacy of intravitreal melphalan for the treatment of RB (Figure 4).29-31
The risk of tumor dissemination after intravitreal injection was evaluated in a 2013 study of 304 patients following therapeutic intravitreal melphalan injections for RB.32 Only one patient had extraocular tumor spread.
 |
The proportion of subjects with extraocular tumor spread potentially due to intravitreal treatment in these combined reports was 0.007 (95% confidence interval, range of 0.0008 to 0.0236), with a mean follow-up of 72.1 months. No reports of tumor spread occurred in a subset of 61 patients receiving intravitreal treatment via safety-enhancing injection techniques (347 injections, 19.6 months mean follow-up). This study concluded that RB metastasis following intravitreal therapy is rare and shouldn’t preclude its clinical use in appropriately selected cases.
Intravitreal chemotherapy safety
Data regarding toxicity of intravitreal melphalan continues to be limited. A 2014 study that evaluated retinal and systemic toxicity of intravitreal melphalan in a rabbit model concluded that weekly injections of 30 µg of melphalan can result in a decreased ERG response.33 Previous studies have also shown that 50-µg of intravitreal melphalan is toxic to the eye with persistent hypotonia and phthisis bulbi.31 In contrast, 20/40 visual acuity has been reported in a patient that received four doses (30, 30, 30 and 20 µg) of intravitreal melphalan with no change in ERG amplitudes before and after therapy.30
 |
Effective intravitreal combination of melphalan (40 µg in 0.04 mL of diluent) and topotecan (8 to 20 µg in 0.04 mL of balanced salt solution) has also recently been reported in nine eyes.34 In the study, no cases of episcleral or orbital retinoblastoma extension or remote retinoblastoma metastasis were reported. There was no change in the A and B waves of bright-flash ERG.
Most recently, a study published this year by Dr. Abramson and colleagues showed that intravitreal chemotherapy may be effective in primary treatment non-vitreous disease, including subretinal seeding, anterior segment dissemination and select retinal tumors.35
Further studies are required to assess the safety of long-term intravitreal therapy and to better delineate its role in the management of retinoblastoma. The adoption of specific guidelines for intravitreal treatment case selection and additional data on potential ocular toxicity remain essential to enable a more widespread use of this treatment.
Periocular chemotherapy
Focal therapies aim at increasing the tumoricidal effects in RB-affected tissues while minimizing systemic toxicity. Multiple researchers have investigated periocular chemotherapy as adjuvant to systemic, intra-arterial and intravitreal chemotherapy.36-39
 |
Treatment-associated toxicities may include transient periorbital edema, strabismus, optic neuropathy, periocular inflammation and fat atrophy. Inflammation associated with periocular agents, particularly carboplatin, has limited their widespread use in children with RB. Periocular topotecan hydrochloride has also been investigated as adjuvant therapy in patients with RB with minimal toxicity.40
A study by one of us (TGM) and colleagues evaluated the effects of intravitreal and subconjunctival melphalan on tumor burden, hypoxia and vasculature in a transgenic mouse retinoblastoma model. We reported a significant decline in hypoxia at one week following intravitreal injection and after maximum dosage of subconjunctival melphalan.41 This study found a significant decrease in tumor burden following serial subconjunctival injections of melphalan, showing an 86 percent reduction. No toxicities were seen on histology following treatments.
Prospective studies are needed to assess the role of periocular chemotherapies for the treatment of RB. Nevertheless, the trend toward direct intravitreal therapy has limited the widespread use of periocular treatments.
 |
Bottom line
Ocular oncology is currently navigating through a therapeutic revolution that is geared toward the application of focal individualized therapies. The majority of these changes in management have happened without clinical trials. Clinical experience remains the most important tool in the management of patients with RB. We anticipate large randomized clinical trials that will better delineate how to use the available therapeutic options more efficiently. RS
REFERENCES
1. Shields CL, Shields JA. Basic understanding of current classification and management of retinoblastoma. Curr Opin Ophthalmol. 2006;17:228–234.
2. Leander C, Fu LC, Pena A, et al. Impact of an education program on late diagnosis of retinoblastoma in Honduras. Pediatr Blood Cancer. 2007;49:817–819.
3. Shields CL, Shields JA. Retinoblastoma management: advances in enucleation, intravenous chemoreduction, and intra-arterial chemotherapy. Curr Opin Ophthalmol. 2010;21:203–212.
4. Shields CL, Santos MC, Diniz W, et al. Thermotherapy for retinoblastoma. Arch Ophthalmol. 1999;117:885–893.
 |
5. Schefler AC, Cicciarelli N, Feuer W, Toledano S, Murray TG. Macular retinoblastoma: evaluation of tumor control, local complications, and visual outcomes for eyes treated with chemotherapy and repetitive foveal laser ablation. Ophthalmology. 2007;114:162–169.
6. Jehanne M, Lumbroso-Le Rouic L, Savignoni A, et al. Analysis of ototoxicity in young children receiving carboplatin in the context of conservative management of unilateral or bilateral retinoblastoma. Pediatr Blood Cancer. 2009;52:637-643.
7. Bartuma K, Pal N, Kosek S, Holm S, All-Ericsson C. A 10-year experience of outcome in chemotherapy-treated hereditary retinoblastoma. Acta Ophthalmol. 2014;92:404-411.
8. De Potter P, Shields CL, Shields JA. Clinical variations of trilateral retinoblastoma: a report of 13 cases. J Pediatr Ophthalmol Strabismus. 1994;31:26–31.
9. Shields CL, Meadows AT, Shields JA, Carvalho C, Smith AF. Chemoreduction for retinoblastoma may prevent intracranial neuroblastic malignancy (trilateral retinoblastoma). Arch Ophthalmol. 2001;119:1269–1272.
10. Shields CL, Mashayekhi A, Au AK, et al. The International Classification of Retinoblastoma predicts chemoreduction success. Ophthalmology. 2006;113:2276–2280.
11. Rodriguez-Galindo C, Wilson MW, Haik BG, et al. Treatment of intraocular retinoblastoma with vincristine and carboplatin. J Clin Oncol. 2003;21:2019–2025.
12. Gombos DS, Kelly A, Coen PG, Kingston JE, Hungerford JL. Retinoblastoma treated with primary chemotherapy alone: The significance of tumour size, location, and age. Br J Ophthalmol. 2002;86:80–83.
13. Shields CL, Mashayekhi A, Cater J, Shelil A, Meadows AT, Shields JA. Chemoreduction for retinoblastoma: Analysis of tumor control and risks for recurrence in 457 tumors. Trans Am Ophthalmol Soc. 2004;102:35–44. discussion 44–35.
14. Schefler AC, Cicciarelli N, Feuer W, Toledano S, Murray TG. Macular retinoblastoma: evaluation of tumor control, local complications, and visual outcomes for eyes treated with chemotherapy and repetitive foveal laser ablation. Ophthalmology. 2007;114:162–169.
15. Honavar SG, Singh AD. Management of advanced retinoblastoma. Ophthalmol Clin North Am. 2005;18:65–73.
16. Eagle RC Jr. High-risk features and tumor differentiation in retinoblastoma: a retrospective histopathologic study. Arch Pathol Lab Med. 2009;133:1203–1209.
17. Honavar SG, Singh AD, Shields CL, et al. Postenucleation adjuvant therapy in high-risk retinoblastoma. Arch Ophthalmol. 2002;120:923–931.
18. Yamane T, Kaneko A, Mohri M. The technique of ophthalmic arterial infusion therapy for patients with intraocular retinoblastoma. Int J Clin Oncol. 2004;9:69–73.
19. Abramson DH, Dunkel IJ, Brodie SE, Kim JW, Gobin YP. A phase I/II study of direct intraarterial (ophthalmic artery) chemotherapy with melphalan for intraocular retinoblastoma initial results. Ophthalmology. 2008; 115:1398–1404. 1404, e1391.
20. Abramson DH, Dunkel IJ, Brodie SE, Marr B, Gobin YP. Superselective ophthalmic artery chemotherapy as primary treatment for retinoblastoma (chemosurgery). Ophthalmology. 2010;17:1623-1629.
21. Shields CL, Manjandavida FP, Lally SE, et al. Intra-arterial chemotherapy for retinoblastoma in 70 eyes: Outcomes based on the international classification of retinoblastoma. Ophthalmology. 2014;121:1453-1460.
22. Mutapcic L, Murray TG, Aziz-Sultan MA, et al. Supraselective intra-arterial chemotherapy: Evaluation of treatment related complications in advanced refractory retinoblastoma. Clin Ophthalmol. In press.
23. Francis JH, Gobin YP, Brodie SE, Marr BP, Dunkel IJ, Abramson DH. Experience of intra-arterial chemosurgery with single agent carboplatin for retinoblastoma. Br J Ophthalmol. 2012;96:1270-1271.
24. Francis JH, Gobin YP, Dunkel IJ, et al. Carboplatin +/- topotecan ophthalmic artery chemosurgery for intraocular retinoblastoma. PLoS One. 2013;8: e72441.
25. Marr BP, Brodie SE, Dunkel IJ, Gobin YP, Abramson DH. Three-drug intra-arterial chemotherapy using simultaneous carboplatin, topotecan and melphalan for intraocular retinoblastoma: preliminary results. Br J Ophthalmol. 2012;96:1300-1303.
26. Gobin YP, Dunkel IJ, Marr BP, Francis JH, Brodie SE, Abramson DH. Combined, sequential intravenous and intra-arterial chemotherapy (bridge chemotherapy) for young infants with retinoblastoma. PLoS One. 2012;7:e44322.
27. Munier FL, Gaillard MC, Balmer A, et al. Intravitreal chemotherapy for vitreous disease in retinoblastoma revisited: From prohibition to conditional indications. Br J Ophthalmol. 2012;96:1078-1083.
28. Francis JH, Abramson DH, Gaillard MC, et al. The classification of vitreous seeds in retinoblastoma and response to intravitreal melphalan. Ophthalmology. 2015;122:1173-1179.
29. Sun YB, Hui P, Punyara K, et al. Intravitreal injection of melphalan in the treatment of retinoblastoma with vitreous cavity seeding. Chin Med J (Engl). 2013;126:1587.
30. Brodie SE, Munier FL, Francis JH, Marr B, Gobin YP, Abramson DH. Persistence of retinal function after intravitreal melphalan injection for retinoblastoma. Doc Ophthalmol. 2013;126:79-84.
31. Ghassemi F, Shields CL. Intravitreal melphalan for refractory or recurrent vitreous seeding from retinoblastoma. Arch Ophthalmol. 2012;130:1268-1271.
32. Smith SJ, Smith BD. Evaluating the risk of extraocular tumour spread following intravitreal injection therapy for retinoblastoma: A systematic review. Br J Ophthalmol. 2013;97:1231-1236.
33. Francis JH, Schaiquevich P, Buitrago E, et al. Local and systemic toxicity of intravitreal melphalan for vitreous seeding in retinoblastoma: A preclinical and clinical study. Ophthalmology. 2014;121:1810-1817.
34. Ghassemi F, Shields CL, Ghadimi H, Khodabandeh A, Roohipoor R. Combined intravitreal melphalan and topotecan for refractory or recurrent vitreous seeding from retinoblastoma. JAMA Ophthalmol. 2014;132:936-941.
35. Abramson DH, Ji X, Francis JH, Catalanotti F, Brodie SE, Habib L. Intravitreal chemotherapy in retinoblastoma: Expanded use beyond intravitreal seeds. Br J Ophthalmol. 2019;103:488-493.
36. Abramson DH, Frank CM, Dunkel IJ. A phase I/II study of subconjunctival carboplatin for intraocular retinoblastoma. Ophthalmology. 1999;106:1947–1950.
37. Leng T, Cebulla CM, Schefler AC, Murray TG. Focal periocular carboplatin chemotherapy avoids systemic chemotherapy for unilateral, progressive retinoblastoma. Retina. 2010;30:S66–68.
38. Mulvihill A, Budning A, Jay V, et al. Ocular motility changes after subtenon carboplatin chemotherapy for retinoblastoma. Arch Ophthalmol. 2003;121:1120–1124.
39. Schmack I, Hubbard GB, Kang SJ, Aaberg TM Jr, Grossniklaus HE. Ischemic necrosis and atrophy of the optic nerve after periocular carboplatin injection for intraocular retinoblastoma. Am J Ophthalmol. 2006;142:310–315.
40. Mallipatna AC, Dimaras H, Chan HS, Héon E, Gallie BL. Periocular topotecan for intraocular retinoblastoma. Arch Ophthalmol. 2011;129:738-745.
41. Shah NV, Pham DG, Murray TG, et al. Intravitreal and subconjunctival melphalan for retinoblastoma in transgenic mice. J Ophthalmol. 2014;2014:829879.
42. Subramanian N, Navaneethakrishnan S, Biswas J, Kanwar RK, Kanwar JR, Krishnakumar S. RNAi mediated Tiam1 gene knockdown inhibits invasion of retinoblastoma. PLoS One. 2013;8:e70422.



